Controlling the building blocks of life

(PhysOrg.com) -- A simple and reliable method for converting one of the simplest chemical entities into one of the most difficult-to-make molecular building blocks of life, with complete control over its shape, is reported by scientists at the University of Bristol in this week's Nature [11 December].
It will have major implications for the synthesis of drugs and agrochemicals.
Many important molecules required for life exist in two forms that are mirror images of each other – like our left and right hands. This property is called ‘chirality’, from the Greek word for hand, and the two forms are called ‘enantiomers’, from the Greek word for opposite.
The classic example of the drug thalidomide illustrates the difference in biological response to chiral molecules: one of the two enantiomers caused devastating birth defects, whereas its mirror image had the desired sedative properties that doctors’ prescribed it for.
Since this catastrophe, and the subsequent recognition of the importance of the relationship between a small molecule (for example a drug) and its site of action (for example a protein), it has become necessary to test individual enantiomers and not mixtures of the two forms. But a mixture of enantiomers can be very difficult to separate.
Professor Varindar Aggarwal at the University of Bristol has now developed a simple and reliable method for converting one of the simplest chemical entities into one of the most difficult-to-make molecular building blocks of life, with complete control over its shape.
Professor Aggarwal explained the importance of this work: “We live in a chiral world. Indeed, chirality and life are so inextricably linked that the detection of chirality outside our planet is used as a test for extraterrestrial life.
“It is the shape and function of a molecule that gives rise to its properties. For example, the different smell of oranges and lemons comes from two molecules, identical except for their three-dimensional spatial arrangement. Thus being able to control the shape and function of enantiomers is critical to the many applications of organic chemical synthesis.”
This work is likely to find broad application in the synthesis of complex organic molecules, particularly in pharmaceuticals and agrochemicals where such difficult shapes are often encountered.
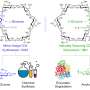
Aggarwal and colleagues have developed a two-step process that can be used to convert readily available secondary alcohols into single mirror image forms of tertiary alcohols that contain a quaternary stereogenic centre (a carbon atom with four different non-hydrogen substituents). Either mirror image of the tertiary alcohol can be made with very high levels of control over its shape.
Provided by University of Bristol