Mysterious nanobubble burst?
(PhysOrg.com) -- The nanobubbles that develop on submerged surfaces should not really be able to exist. Because of the enormous internal pressure, they should disappear within a short time. Nevertheless, they sometimes last for hours: an unexplained phenomenon.
Professor Detlef Lohse of the University of Twente and his colleague Professor Michael Brenner of Harvard have, however, revealed something of interest. They demonstrated that an equilibrium can develop between the gas that leaves the bubble and the gas that flows into it. It is even possible to calculate the dimensions of a bubble in which this happens. The researchers’ work is being published in Physical Review Letters at the end of November.
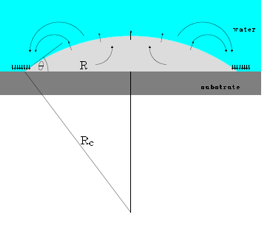
The fact that bubbles can develop on a water-repellent surface, submerged in water, had already been demonstrated: these are the round ‘caps’ (see figure) with a diameter of about 100 nanometres and a height of 10 nanometres. The reason they develop is still a mystery but they are nevertheless useful: for example, liquids flow more easily, more rapidly and with less energy consumption along surfaces covered with bubbles. The first techniques for stimulating bubble formation have already been developed as well.
Equilibrium
Nevertheless, it is frustrating that there is still no explanation of how nanobubbles exist. Why should they develop? Small gas bubbles should dissolve rapidly because of the immense internal pressure, the gas flowing out of the bubble. They should disappear within microseconds, whereas measurements have shown that they can last for hours. Lohse and Brenner are searching for the reason why gas flows out of the bubble and, at the same time, inwards. When the two forces are in equilibrium, the bubble can remain intact for much longer than was first thought possible.
According to their theory, the inward flow takes place at the edge of the bubble; in other words, where the edge of a bubble comes in contact with a hydrophobic surface. It is known that, close to a hydrophobic surface, there is a higher concentration of gas molecules: these are then attracted by the surface. If these molecules now flow in via the edge of a bubble, they can reach a state of equilibrium with the molecules that are coming out of the bubble. This equilibrium is actually unstable: according to the second law of thermodynamics, this should only be a transitional phase, implying that the bubbles will dissolve within hours or perhaps days.
The theory, presented in Physical Review Letters, explains the long life of the bubbles. However, the researchers would like to look further into the long-term behaviour of these bubbles. Is the equilibrium unstable after all? Besides this, the new insights help with artificial stimulation of bubbles at the surface, for example, by means of electrolysis.
More information: link.aps.org/abstract/PRL/v101/e214505
Provided by University of Twente, Netherlands