Nanoparticles Target Multiple Cancer Genes, Shrink Tumors More Effectively
(PhysOrg.com) -- Nanoparticles filled with small interfering RNA (siRNA) molecules targeting two genes that trigger melanoma have shown that they can inhibit the development of melanoma, the most dangerous type of skin cancer. The nanoparticles, administered in conjuction with ultrasound irradiation, exerted their effects only on malignant tissue, leaving healthy tissue alone.
“It is a very selective and targeted approach,” said Gavin Robertson, Ph.D., who led the team of researchers from the Penn State College of Medicine. “And unlike most other cancer drugs that inadvertently affect a bunch of proteins, we are able to knock out single genes.”
The Penn State researchers speculated that siRNA could turn off the two cancer-causing genes and potentially treat the deadly disease more effectively. “siRNA checks the expression of the two genes, which then lowers the abnormal levels of the cancer causing proteins in cells,” explained Dr. Robertson. This research appears in the journal Cancer Research.
In recent years, researchers have zeroed in on two key genes—B-Raf and Akt3—that play key roles in the development of melanoma. Mutations in the B-Raf gene, the most frequently mutated gene in melanoma, lead to the production of a mutant form of the B-Raf protein, which then helps mole cells survive and grow. B-Raf mutations alone, however, do not trigger melanoma development. That event requires a second protein, called Akt3, that regulates the activity of the mutated B-Raf, which aids the development of melanoma. The siRNA agents used in this study specifically target Akt3 and the mutant B-Raf and therefore do not affect normal cells.
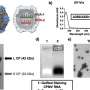
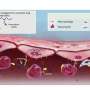
However, although knocking out specific genes may seem like a straightforward task, delivering the siRNA drug to cancerous cells is another story, because not only do protective layers in the skin keep drugs out but also chemicals in the skin quickly degrade the siRNA. To clear these two hurdles, Dr. Robertson and his team engineered lipid-based nanoparticles that can incorporate siRNA into their hollow interiors. The researchers then used a portable ultrasound device to temporarily create microscopic holes in the surface of the skin, allowing the drug-filled particles to leak into tumor cells beneath.
When the researchers exposed lab-generated skin containing early cancerous lesions to the treatment 10 days after the skin was created, the siRNA reduced the ability of cells containing the mutant B-Raf to multiply by nearly 60 to 70 percent and more than halved the size of lesions after 3 weeks. “This is essentially human skin with human melanoma cells, which provides an accurate picture of how the drug is acting,” said Dr. Robertson.
Mice with melanoma that underwent the same treatment had their tumors shrink by nearly 30 percent when only the mutant B-Raf was targeted. There was no difference in the development of melanoma when the Akt3 gene alone was targeted, although existing tumors shrank by about 10 to 15 percent in 2 weeks. However, when the researchers targeted both Akt3 and mutant B-Raf at the same time, they found that tumors in the mice shrank about 60 to 70 percent more than when either gene was targeted alone.
“If you knock down each of these two genes separately, you are able to reduce tumor development somewhat,” Dr. Robertson said. “But knocking them down together leads to synergistic reduction of tumor development.”
This work, which was supported in part by the National Cancer Institute, was detailed in the paper “Targeting V600EB-Raf and Akt3 Using Nanoliposomal-Small Interfering RNA Inhibits Cutaneous Melanocytic Lesion Development.” An abstract of this paper is available at the journal’s Web site (dx.doi.org/doi:10.1158/0008-5472.CAN-07-6614).
Provided by National Cancer Institute