New solar energy material captures every color of the rainbow
Researchers have created a new material that overcomes two of the major obstacles to solar power: it absorbs all the energy contained in sunlight, and generates electrons in a way that makes them easier to capture.
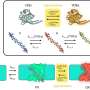
Ohio State University chemists and their colleagues combined electrically conductive plastic with metals including molybdenum and titanium to create the hybrid material.
"There are other such hybrids out there, but the advantage of our material is that we can cover the entire range of the solar spectrum," explained Malcolm Chisholm, Distinguished University Professor and Chair of the Department of Chemistry at Ohio State.
The study appears in the current issue of the Proceedings of the National Academy of Sciences (PNAS).
Sunlight contains the entire spectrum of colors that can be seen with the naked eye -- all the colors of the rainbow. What our eyes interpret as color are really different energy levels, or frequencies of light. Today's solar cell materials can only capture a small range of frequencies, so they can only capture a small fraction of the energy contained in sunlight.
This new material is the first that can absorb all the energy contained in visible light at once.
The material generates electricity just like other solar cell materials do: light energizes the atoms of the material, and some of the electrons in those atoms are knocked loose.
Ideally, the electrons flow out of the device as electrical current, but this is where most solar cells run into trouble. The electrons only stay loose for a tiny fraction of a second before they sink back into the atoms from which they came. The electrons must be captured during the short time they are free, and this task, called charge separation, is difficult.
In the new hybrid material, electrons remain free much longer than ever before.
To design the hybrid material, the chemists explored different molecular configurations on a computer at the Ohio Supercomputer Center. Then, with colleagues at National Taiwan University, they synthesized molecules of the new material in a liquid solution, measured the frequencies of light the molecules absorbed, and also measured the length of time that excited electrons remained free in the molecules.
They saw something very unusual. The molecules didn't just fluoresce as some solar cell materials do. They phosphoresced as well. Both luminous effects are caused by a material absorbing and emitting energy, but phosphorescence lasts much longer.
To their surprise, the chemists found that the new material was emitting electrons in two different energy states -- one called a singlet state, and the other a triplet state. Both energy states are useful for solar cell applications, and the triplet state lasts much longer than the singlet state.
Electrons in the singlet state stayed free for up to 12 picoseconds, or trillionths of a second -- not unusual compared to some solar cell materials. But electrons in the triplet state stayed free 7 million times longer -- up to 83 microseconds, or millionths of a second.
When they deposited the molecules in a thin film, similar to how they might be arranged in an actual solar cell, the triplet states lasted even longer: 200 microseconds.
"This long-lived excited state should allow us to better manipulate charge separation," Chisholm said.
At this point, the material is years from commercial development, but he added that this experiment provides a proof of concept -- that hybrid solar cell materials such as this one can offer unusual properties.
Source: Ohio State University