Putting the Squeeze on Nitrogen for High Energy Materials
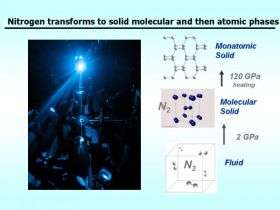
(PhysOrg.com) -- Nitrogen atoms like to travel in pairs, hooked together by one of the strongest chemical bonds in nature. By subjecting nitrogen molecules to extreme temperatures and pressures scientists are getting a new understanding of not only nitrogen but other similar molecules, including hydrogen. In the current online edition of Physical Review Letters, researchers from the Carnegie Institution's Geophysical Laboratory report changes in the melting temperature of solid nitrogen at pressures up to 120 gigapascals (more than a million atmospheres) and temperatures reaching 2,500° Kelvin (more than 4000° Fahrenheit).
These results, plus observed changes in the structure of solid nitrogen at high pressures, could lead to new high energy nitrogen- or hydrogen-based fuels in the future. Hypothesized nitrogen polymers could form materials with higher energy content than any known non-nuclear material.
Alexander Goncharov, Viktor Struzhkin, and Russell Hemley from Carnegie, with Jonathan Crowhurst from Lawrence Livermore National Laboratory, compressed liquid nitrogen in a device known as a diamond anvil cell, which generates ultrahigh pressures by squeezing a sample between two gem-quality diamonds. Because the diamonds are transparent to most wavelengths of light, the sample can be heated by a laser during the experiment. A technique called Raman spectroscopy uses light emitted by the heated sample to analyze changes in the sample's molecular structure as they occur.
"Until now, no one had made these kinds of in situ observations of nitrogen at such extreme temperatures and pressures," says Goncharov. "Our measurements of the melting line and the vibration properties of the fluid indicated by the Raman spectroscopy give us a very clear picture of how nitrogen and its molecular bonds respond under these conditions."
A chart of the temperatures and pressures at which a substance changes from one phase to another (from liquid to gas, from one crystal structure to another, and so on) is called a phase diagram. For nitrogen, as well as most other materials, the high temperature and pressure regions of the phase diagram are relatively unexplored territories. Researchers hope that these unexplored regions may harbor new materials with useful properties.
At room temperature and atmospheric pressure, nitrogen is a gas, but it can be cooled and compressed to form a liquid or a solid, depending on the temperature and pressure. Even as it changes phases, however, the nitrogen remains a two atom (diatomic) molecule, held together by a strong—and energy rich—triple bond.
"Nitrogen compounds tend to be high energy density materials," says Goncharov. "Pure nitrogen can be a powerful fuel or explosive if one can figure out how to associate nitrogen atoms in a material other than as a triple-bonded diatomic molecule. Recent experiments have shown that nitrogen transforms to nonmolecular single-bonded phases at very high pressure. These could serve as high energy density materials if preserved on a return to ambient pressure. Our results will help show the way to synthesize these materials at less extreme conditions."
Provided by Carnegie Institution