Explosives go 'green'
(Physorg.com) -- Certain explosives may soon get a little greener and a little more precise. LLNL researchers added unique green solvents (ionic liquids) to an explosive called TATB (1,3,5-triamino-2,4,6-trinitrobenzene) and improved the crystal quality and chemical purity of the material.
This work, supported under the Transformational Materials Initiative (TMI) Laboratory Research and Development project, appears on the cover of the Sept. 1 issue of the journal Physical Chemistry Chemical Physics.
"Improving crystal quality and purity leads to explosive materials that are safer (less likely to react violently) when subjected to mechanical impact or heat," said Larry Fried, the project's principal investigator and a co-author of the paper.
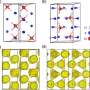
Most explosives belong to a general class of materials called molecular crystals, which have become important building blocks in a number of other applications ranging from drugs, pigments, agrochemicals, dyes and optoelectronics. Many of these materials, including TATB, are bound together by a strong network of hydrogen-bonds. This extended network often makes these materials nearly insoluble in common organic solvents, leading to poor quality and limited size crystals, which in turn hinders progress in many technological applications.
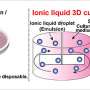
So the TMI team looked for a suitable alternative, which happened to be ionic liquids - a special type of molten salt that becomes liquid under the boiling point of water (100 degrees Celsius). Chemists are recently interested in ionic liquids because they are solvents with almost no vapor pressure, and do not evaporate, even under high temperature conditions. They also provide researchers an endless number of choices due to the large combinations of positive and negative ions involved.
To narrow the choices down, lead author Amitesh Maiti used state-of-the-art quantum mechanical simulations to identify a special class of ionic liquids containing fluoride anions that are highly effective in dissolving hydrogen-bonded materials such as TATB. (An anion is an atom with more electrons than protons in its nuclei.)
"The design of custom solvents through first principles modeling opens up new possibilities for the dissolution of materials that are hard to dissolve," Maiti said.
The next step involved an experimental team, led by Phil Pagoria, who was successful not only in dissolving TATB in such solvents, but in growing large defect-free crystallites (more than 97 percent pure TATB), which will lead to a better formulated material for explosive applications.
The solvents and the dissolution process developed by the TMI team have applications in other fields as well, such as the production of polymers (plastics) or molecular solids (pharmaceuticals, paints, propellants, explosives). For instance, the team found that fluoride ionic liquids are highly effective in dissolving cellulose (plant fiber), a versatile bio-renewable polymeric material with many applications.
However, the immediate goal is to find a cost-effective way to improve the quality of low purity TATB. TATB is an extremely safe explosive that is used by the Department of Energy, the Department of Defense and the mining industry.
Source: Lawrence Livermore National Laboratory