Molecular bridge serves as a tether for a cell's nucleus
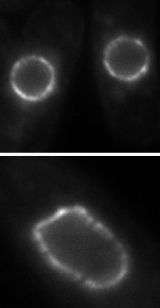
(PhysOrg.com) -- A cell's nucleus - home of it its most precious contents — is a delicate envelope that, without support, is barely able to withstand the forces that keep it in place. Now, researchers have discovered a network of molecules in the nuclear membrane that provide the nucleus with rigidity and also facilitate a previously undiscovered form of communication between the cell’s nucleus and its cytoplasm.
In the August 8 issue of Cell, the scientists, led by Nobel Prize winner Gьnter Blobel, say that this mechanism is different from the usual traffic of molecular signals that enter and exit the nucleus through pores in the nuclear envelope.
“This is a distinct kind of physical connection between two compartments in a cell — the cytoplasm and the nucleus,” says the study’s lead investigator, Megan King, of Blobel’s Laboratory of Cell Biology. “It really opens up the possibility that there is a basic process going on that affects gene expression in ways that we had not understood before.”
King describes the network as a bridge of molecules that extends from the interior of the nucleus — specifically, the chromatin, the complex of DNA and proteins that makes up chromosomes – into the cell cytoplasm and its network of microtubules that provides structure to the cell. Though some of the proteins had been previously identified, King, Blobel and researcher Theodore Drivas discovered one in particular, called Ima1, that serves as one of the bridge’s pillars.
In fission yeast, the single-cell eukaryotes that the researchers used as a model organism, the nucleus has to stay centered within the cell before cell division is initiated. In other eukaryotes, microtubules push on the nucleus by interacting with nuclear membrane proteins from two previously discovered families: KASH domain proteins, which span the outer nuclear membrane, and SUN domain proteins, which reside in the inner nuclear membrane. However, King and the other researchers suspected that this bridge alone could not be strong enough to keep the nuclear structure stable against the forces applied by the cell’s cytoskeleton.
The researchers now say that two additional proteins are part of the yeast nuclear bridge. One is Kms2, which is part of the KASH family, and the other is Sad1, a member of the SUN family. Kms2 forms the outside pillar of the bridge and couples forces from microtubules to the protein bridge anchored on the inside by Sad1. But the scientists suspected there had to be an anchor for Sad1, or the bridge could not withstand such forces.
They then examined Ima1, which is found in many species, including humans. The protein binds to heterochromatin, which is a tightly packed form of DNA and is critically located in the inner membrane of the nucleus. Indeed, a series of experiments demonstrated that Ima1 forms the strong ground support for the side of the bridge that attaches inside the nucleus, and that other proteins, namely the Ndc80 complex, strengthen the connection, like a nut-and-bolt arrangement. Together, they proved able to absorb the forces transmitted through the centrosome on the outside of the nucleus. Whenever Ima1 or the Ndc80 complex was compromised, the bridges fell apart.
“The proteins act like players in a game of tug-of-war,” says King. “They will move side to side in an ordered line, remaining standing as long as the teams are of similar strengths. However, once one team pulls with a force that cannot be countered by the second team, both teams fall to the ground in a jumble.” When decoupled from chromatin, the nuclear envelope pays the price, becoming deformed and fragmented.
“This communication is physical, and it shows us how chromatin can support a cytoplasmic function, while, on the other hand, microtubules have the ability to affect nuclear functions,” King says.
Citatoin: Cell 134(3): 427–438 (August 8, 2008)
Provided by Rockefeller University