Controlling the Size of Nanoclusters: First Step in Making New Catalysts
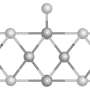
Researchers from the U.S. Department of Energy's Brookhaven National Laboratory and Stony Brook University have developed a new instrument that allows them to control the size of nanoclusters — groups of 10 to 100 atoms — with atomic precision. They created a model nanocatalyst of molybdenum sulfide, the first step in developing the next generation of materials to be used in hydrodesulfurization, a process that removes sulfur from natural gas and petroleum products to reduce pollution.
As reported in the July 9, 2008 online edition of the Journal of Physical Chemistry C, the scientists made size-selected molybdenum sulfide nanoclusters as gaseous ions, and then gently deposited the clusters on a gold surface. The nanoclusters interact weakly with the gold support and therefore remain intact.
"With this new instrument, we can control how many and what type of atoms are in a nanocluster," said Brookhaven chemist Michael White, the principal author of the paper. "This knowledge enables us to make nanoclusters with predetermined size, structure and chemical composition, all which are important for the design of new catalysts."
Currently, molybdenum sulfide nanoparticles are used for hydrodesulfurization and other chemical processes, but it is not known what size is most active or how the reactions occur on small particles. The ability to make model nanocatalysts containing molybdenum sulfide particles of variable size and chemical makeup will allow White and coworkers to understand how current catalysts work and help design the next generation of catalysts.
In the current research, the scientists explored the chemical reactivity of a very stable or “magic” cluster of four atoms of molybdenum and six atoms of sulfur deposited on a gold surface. This small nanocluster is thought to be prototypical of active catalyst particles because all the molybdenum metal atoms are exposed and therefore can react with other molecules. Exploring larger and more reactive nanoclusters will be the next step.
"This was a study to test the capabilities of the newly built instrument," White said. "Now we can do further studies with different nanoclusters to find those that are most reactive and therefore best suited as models for making the most efficient nanocatalysts."
Melissa Patterson, a W. Burghardt Turner Fellow at Stony Brook University and a coauthor of the paper, will give a talk on related work titled "Size-selected deposition of transition metal sulfides: Insights toward model systems in catalysis" at the American Chemical Society's national meeting in Philadelphia on August 19, 2008.
Source: Brookhaven National Laboratory