New nano technique significantly boosts boiling efficiency
Whoever penned the old adage "a watched pot never boils" surely never tried to heat up water in a pot lined with copper nanorods.
A new study from researchers at Rensselaer Polytechnic Institute shows that by adding an invisible layer of the nanomaterials to the bottom of a metal vessel, an order of magnitude less energy is required to bring water to boil. This increase in efficiency could have a big impact on cooling computer chips, improving heat transfer systems, and reducing costs for industrial boiling applications.
"Like so many other nanotechnology and nanomaterials breakthroughs, our discovery was completely unexpected," said Nikhil A. Koratkar, associate professor in the Department of Mechanical, Aerospace, and Nuclear Engineering at Rensselaer, who led the project. "The increased boiling efficiency seems to be the result of an interesting interplay between the nanoscale and microscale surfaces of the treated metal. The potential applications for this discovery are vast and exciting, and we're eager to continue our investigations into this phenomenon."
Bringing water to a boil, and the related phase change that transforms the liquid into vapor, requires an interface between the water and air. In the example of a pot of water, two such interfaces exist: at the top where the water meets air, and at the bottom where the water meets tiny pockets of air trapped in the microscale texture and imperfections on the surface of the pot. Even though most of the water inside of the pot has reached 100 degrees Celsius and is at boiling temperature, it cannot boil because it is surrounded by other water molecules and there is no interface — i.e., no air — present to facilitate a phase change.
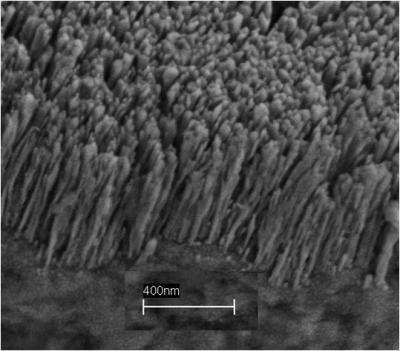
Bubbles are typically formed when air is trapped inside a microscale cavity on the metal surface of a vessel, and vapor pressure forces the bubble to the top of the vessel. As this bubble nucleation takes place, water floods the microscale cavity, which in turn prevents any further nucleation from occurring at that specific site.
Koratkar and his team found that by depositing a layer of copper nanorods on the surface of a copper vessel, the nanoscale pockets of air trapped within the forest of nanorods "feed" nanobubbles into the microscale cavities of the vessel surface and help to prevent them from getting flooded with water. This synergistic coupling effect promotes robust boiling and stable bubble nucleation, with large numbers of tiny, frequently occurring bubbles.
"By themselves, the nanoscale and microscale textures are not able to facilitate good boiling, as the nanoscale pockets are simply too small and the microscale cavities are quickly flooded by water and therefore single-use," Koratkar said. "But working together, the multiscale effect allows for significantly improved boiling. We observed a 30-fold increase in active bubble nucleation site density — a fancy term for the number of bubbles created — on the surface treated with copper nanotubes, over the nontreated surface."
Boiling is ultimately a vehicle for heat transfer, in that it moves energy from a heat source to the bottom of a vessel and into the contained liquid, which then boils, and turns into vapor that eventually releases the heat into the atmosphere. This new discovery allows this process to become significantly more efficient, which could translate into considerable efficiency gains and cost savings if incorporated into a wide range of industrial equipment that relies on boiling to create heat or steam.
"If you can boil water using 30 times less energy, that's 30 times less energy you have to pay for," he said.
The team's discovery could also revolutionize the process of cooling computer chips. As the physical size of chips has shrunk significantly over the past two decades, it has become increasingly critical to develop ways to cool hot spots and transfer lingering heat away from the chip. This challenge has grown more prevalent in recent years, and threatens to bottleneck the semiconductor industry's ability to develop smaller and more powerful chips.
Boiling is a potential heat transfer technique that can be used to cool chips, Koratkar said, so depositing copper nanorods onto the copper interconnects of chips could lead to new innovations in heat transfer and dissipation for semiconductors.
"Since computer interconnects are already made of copper, it should be easy and inexpensive to treat those components with a layer of copper nanorods," Koratkar said, noting that his group plans to further pursue this possibility.
Source: Rensselaer Polytechnic Institute




















