Quasicrystal mystery unraveled with computer simulation
The method to the madness of quasicrystals has been a mystery to scientists. Quasicrystals are solids whose atoms aren't arranged in a repeating pattern, as they are in ordinary crystals. Yet they form intricate patterns that are technologically useful.
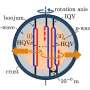
A computer simulation performed by University of Michigan scientists has given new insights into how this unique class of solids forms. Quasicrystals incorporate clusters of atoms as they are, without rearranging them as regular crystals do, said Sharon Glotzer, a professor in the Department of Chemical Engineering.
Crystals form when liquids freeze into solids. When a normal crystal grows, a crystallite nucleus develops first. The atoms in the liquid attach one-by-one to the crystallite, as though following a template. If the atoms have already formed a cluster on their own, they must rearrange in order to fit the template. This is how a repeating pattern forms.
In the case of quasicrystals, though, atoms that have already formed stable shapes away from the crystallite can still bind to it. They don't have to make adjustments.
"In our simulations of quasicrystals, we observed that the atoms attach to the crystallite in large groups," said chemical engineering doctoral student Aaron Keys. "These groups have already formed locally stable arrangements, and the growing quasicrystal assimilates them with minimal rearrangement."
Because quasicrystals aren't as regimented as regular crystals, the solid can reach a "structural compromise," where liquid-like molecular arrangements are retained in the solid state. This allows quasicrystals to form more easily than regular crystals.
Quasicrystals are found in certain metal alloys that tend to resist wear and corrosion, and are used in non-stick coatings, for example. They also have high tensile strength, meaning high forces are required to stretch them to their breaking point.
"Learning how they grow will help us figure out to how engineer quasicrystalline structures from new building blocks, which could lead to a slew of new materials," Glotzer said.
Glotzer and Keys are authors of a paper on the research, "How do quasicrystals grow?," published in Physical Review Letters. Their paper is featured in an article in the current edition of the journal Nature.
Source: University of Michigan