November 15, 2007 feature
First Direct Images of Carbon Nanotubes Entering Cells
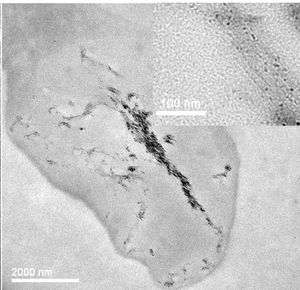
For the first time, scientists have directly imaged carbon nanotubes entering and migrating within human cells, determining as a result that whether the nanotubes cause cell death depends on the dose and exposure time. The work, published in the October 28 online edition of Nature Nanotechnology, may lead to better ways of determining carbon nanotubes' toxicity to humans.
This study is the first to show definitively that carbon nanotubes have the ability to cross into the cytoplasm and nucleus of a cell.
Many studies have explored the toxicity of carbon nanotubes, some concluding that the nanotubes are acutely toxic and some not. But the uptake of carbon nanotubes by cells has never before been directly observed, casting doubt on the accuracy of those studies.
“Contradictory data on the toxic effects of single-walled carbon nanotubes highlight the need for alternative ways to study their uptake and cytotoxic effects in cells,” lead scientist Alexandra Porter, of the University of Cambridge in the UK, said to PhysOrg.com. “But the direct observation of cellular uptake of single-walled carbon nanotubes has been hindered by difficulties in discriminating carbon-based nanotubes from carbon-rich cell structures.”
Porter and her colleagues from the University of Cambridge and Daresbury Laboratory, also in the UK, used two types of microscopy to image single-walled carbon nanotubes as they entered macrophages, which are cells of the human immune system. They “watched” the nanotubes enter the cytoplasm, certain organelles, and the nuclei.
The group chose macrophages because they are the first line of defense against foreign materials in many tissues in the body, including lung tissue. Nanoparticles that are inhaled, such as carbon nanotubes in powder form, should be ingested by macrophages, which may stop the nanotubes from getting further into the body’s system, such as the blood and lymph systems (lymph is a clear fluid containing white blood cells and tissue waste that is part of the body's defense against infection).
The cells were analyzed both stained and unstained and the results compared to two common viability tests, or “assays.” These assays gauge the health of the cells using different markers, and thus can have different results. On the other hand, the imaging techniques the scientists used determine cell death by allowing clearly defined structural changes to be identified, and could therefore become an essential complement to carbon-nanotube toxicity assays.
The cells analyzed were treated with nanotube solutions over time periods of two and four days at concentrations between zero (no nanotubes) and 10 micrograms (millionths of a gram) per milliliter.
The images revealed that even cells subjected to the highest nanotube concentrations were still relatively healthy after two days; there were no major differences between the control cells, which were not treated, and the nanotube-treated cells. But after four days, even the lower concentrations led to a significant decrease in the cells' viability.
After two days, the nanotubes had entered the cells' lysosomes, organelles in the cytoplasm that cause the breakdown of metabolic substances and foreign particles within the cell. After four days, the nanotubes had fused together; some had entered the cytoplasm and crossed into the nucleus.
“Uptake to these sites implies that the nanotubes may interact with intracellular proteins, organelles, and DNA, which would greatly enhance their toxic potential,” said Porter.
The two imaging techniques used are transmission electron microscopy (TEM) and confocal microscopy. In TEM, a beam of electrons passes through a thin specimen, forming an image that is recreated on a fluorescent screen, photographic film, or detected by a camera. Confocal microscopy involves the use of light to increase the contrast of an image by eliminating all light that is not in the focal plane of the image, resulting in a much sharper image.
Citation: Alexandra E. Porter, Mhairi Gass, Karin Muller, Jeremy N. Skepper, Paul A. Midgley and Mark Well Nature Nanotechnology advance online publication, 28 October 2007 (doi:10.1038/nnano2007.347)
Copyright 2007 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.





















