January 7, 2016 report
Scientists determine the structure of Titan's evaporites
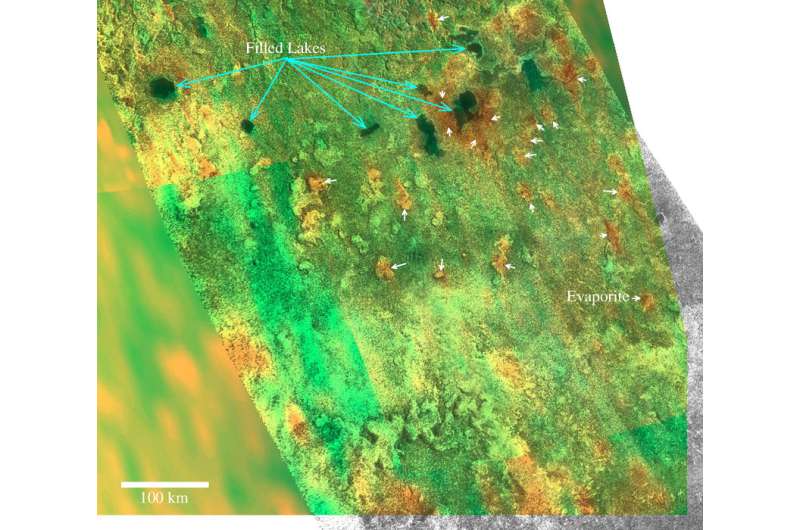
(Phys.org)—Titan, the largest moon of Saturn, hosts many interesting geological features that could be evaporites. While the chemical compositions of these sediments have been studied by scientists, their structure still remains a puzzle. Now, an international team of researchers using available laboratory measurements has created models to explore the structure of evaporites on Titan. A paper detailing their study appeared online on Dec. 22 in the arXiv journal.
The NASA/ESA Cassini spacecraft, studying Saturn and its moons since 2004, is an invaluable source of important information about Titan and its geological features. The probe's on-board radar instrument investigates the surface of Titan by taking four types of observations: imaging, altimetry, backscatter, and radiometry. These observations are crucial to understanding the geology of this hazy, cold moon and the topography of its solid surface.
The models—developed by a team of scientists led by Daniel Cordier of the University of Reims Champagne-Ardenne, France—could be the most important tools for investigating geologic formations on Titan. They confirm the possibility of an acetylene- or butane-enriched central layer of evaporitic deposit. The computations indicate that an accumulation of poorly soluble species like hydrogen cyanide or aerosols similar to tholins could play a dominant role. The models also predict the existence of chemically trimodal "bathtub rings." Moreover, the research offers an explanation to the lack of evaporites in the south polar region of Titan and to the high radar reflectivity of dry lakebeds.
"The existence of a hydrologic cycle based on methane and the presence of other, more complex, hydrocarbons open the possibility of the occurrence of evaporite formation at the surface of Titan," Cordier told Phys.org.
Titan hosts lakes and seas likely filled by liquid hydrocarbons containing some amount of dissolved atmospheric nitrogen and various organic compounds. The formation of any evaporite layer requires a sequence of wet and dry periods. During the wet episode, methane and ethane rains dissolve the solid organics encountered along their runoff at the ground, and then finally they fill the lacustrine depressions. The dry period produces the evaporation of the solvents and the formation of evaporates. The sequence of dry-wet periods can span over a single year if driven by Titan's seasonal effect. Alternatively, the formation process of evaporites, described in previous papers by Jason Barnes and Shannon MacKenzie, could be the consequence of climate change over much longer timescales.
"Very little is known about these structures. First of all, due to the thick atmosphere, it is very difficult to derive information about Titan's surface composition. Jason Barnes and Shannon MacKenzie have found correlations between zones poor in water ice and dry lakebeds detected by the Cassini radar. Of course, the existence of a hydrologic cycle based on methane and the presence of other, more complex, hydrocarbons open the possibility of the occurrence of evaporite formation at the surface of Titan," Cordier explained.
Cordier and his colleagues have computed the possible vertical structure of evaporite deposits. Their simulations confirm that butane and acetylene are good candidates for species that could compose the surface of evaporite. They found that a couple of compounds could form a thick external layer; due to the combination of the existence of two crystallographic phases and of the rather thick layer, this external butane-acetylene-enriched layer could explain the radar brightness of evaporites.
The researchers have also shown in the study that the seasonal cycle may offer a mechanism which leads to a growth of evaporate thickness only limited by atmospheric production of organics. Their calculation suggests that Titan's ethane-enriched south pole lake, Ontario Lacus, could have trapped a large quantity of solutes, explaining the lack of evaporites in the south polar regions of this moon. The models also confirm the possibility of the formation of "bathtub rings" showing a complex chemical composition and the possible existence of trimodal "bathtub ring" compositions when the evaporation is completed.
Cordier underlined the need for a future space mission to Titan involving a lander focused partly on the exploration of the lakes shores, where the chemical diversity is clearly high. Sending a spacecraft could advance our knowledge about Titan's evaporites and its interesting geological features.
"The perfect appropriate probe to explore these terrains would be an amphibian rover equipped with a drill. This kind of robot could allow the exploration of both evaporitic terrains around a partially filled lake and the liquid phase itself. But this kind of project belongs to the future," Cordier concluded.
More information: Structure of Titan's evaporites, arXiv:1512.07294 [astro-ph.EP] arxiv.org/abs/1512.07294. arxiv.org/abs/1512.07294
Abstract
Numerous geological features that could be evaporitic in origin have been identified on the surface of Titan. Although they seem to be water-ice poor, their main properties -chemical composition, thickness, stratification- are essentially unknown. In this paper, which follows on a previous one focusing on the surface composition (Cordier et al., 2013), we provide some answers to these questions derived from a new model. This model, based on the up-to-date thermodynamic theory known as "PC-SAFT", has been validated with available laboratory measurements and specifically developed for our purpose. 1-D models confirm the possibility of an acetylene and/or butane enriched central layer of evaporitic deposit. The estimated thickness of this acetylene-butane layer could explain the strong RADAR brightness of the evaporites. The 2-D computations indicate an accumulation of poorly soluble species at the deposit's margin. Among these species, HCN or aerosols similar to tholins could play a dominant role. Our model predicts the existence of chemically trimodal "bathtub rings" which is consistent with what it is observed at the south polar lake Ontario Lacus. This work also provides plausible explanations to the lack of evaporites in the south polar region and to the high radar reflectivity of dry lakebeds.
© 2016 Phys.org




















