August 27, 2015 report
Neural qubits: Quantum cognition based on synaptic nuclear spins
(Phys.org)—The pursuit of an understanding of the base machinery of the mind led early researchers to anatomical exhaustion. With neuroscience now in the throes of molecular mayhem and a waning biochemical bliss, physics is spicing things up with a host of eclectic quantum, spin, and isotopic novelties. While increases in electron spin content have been linked to anesthetic effects, nuclear spins have recently been implicated in a more rarefied and subtle phenomenon— neural quantum processing.
Matthew Fisher from University of California at Santa Barbara has hashed out one scenario by which it all could work in a new paper now on the Arxiv server. He notes that while small molecules and ions would rapidly entangle with a surrounding wet environment and therefore couldn't maintain quantum coherence on macroscopic time scales, nuclear spins are exceptional in being so weakly coupled to environmental degrees of freedom that prolonged phase coherence is likely.
How long, you might ask? That depends which element you are talking about, and the quantum value of its spin. Every element has a nucleus that can be characterized by half-integer spin-magnitude (I = 0; 1=1/2; 1...). Quantum decoherence of the nuclear spin is caused by magnetic and electric field perturbations which effectively kill any hope for quantum processing. Fisher claims that the element with the optimal coherence time (Tcoh), and therefore the one ideally poised to host the putative neural qubit should have nuclear spin of 1/2. In a biochemical setting, spin 1/2 nuclei are weakly decohered only by magnetic fields while for spin >1/2 electric fields cause large decoherence. Spin 0 nuclei lack any associated magnetic dipole moment interaction with nuclear magnetic fields
Fisher's interest in neural nuclear spin processing was stimulated by a paper that explored the effects of different isotopes of lithium on rats. Li naturally occurs in the ratio 92.6% Li-7 and 7.4% Li-6. Somewhat quizzically, mothers given the Li-7 isotope were less stimulated and ignored their pups while the Li-6 moms were maternalistic and nursed more. The interesting part for us here, is that while Li-7 has spin 3/2 and a short Tcoh of just a few seconds, Li-6 has an "honorary" spin 1/2 due to its electric dipole moment and a nice 5 minute long Tcoh.
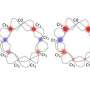
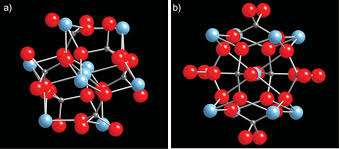
Since Li isn't common to most life, and because phosphorus is the only common biochemical element that has spin 1/2, Fisher focused on organophosphates. These are the various common esters of inorganic phoshopate ions (Pi) like ATP and poly-phosphate chains (PPi). Although these froms of phosphate have a Tcoh on the order of a second and might serve as effective neural qubit transporters in the right context, Fisher theorized that a form of phosphate known as a Posner molecule might better serve for qubit memory storage. If calcium displaces the proton in phosphate ions, Posner clusters of Ca9(PO4)6 have significantly longer Tcoh times (potentially days) can form. Previous x-ray diffraction studies have even suggested that amorphous calcium phosphate Posner clusters could be a key step in the mineralization of bone into hydroxyapaptite crystal.
The present discussion of regarding curious phosphate phenomena does not pop out fully-formed from in its own void. There is an ample if occaisionally controversial body of experimental findings dealing with magnetic isotope and field effects on the major phosphorylation reactions of life Regarding the enzyme catalyzed hydrolysis reaction of PPi into Pi + Pi, Fisher outlines a scenario whereby reaction rate is dependent on the nuclear spin state, ie. different for the singlet and triplet states. To pool ideas together under the rubric of actually performing quantum processing, some form of quantum entanglement and subsequent measurement is needed.
To get there we need to imagine entangled phosphate pairs released into extracellular fluid, as at a synapse, where they can combine with calcium ions to form multiple Posner molecules that in effect hold entangled phosphorus spins in memory. The magic happens if and when Posner molecules bind after being transported into two separate presynaptic terminals. At that point, the paper claims, they would be suseptible to melting in the acidified interior of the vesicle through which they entered, with subsequent synaptic calcium dynamics and entangled postsynaptic firing. The stability of Posner molecules appears to be exquisitely pH-sensitive in bone, and by implication elsewhere. Binding (into dimers and higher order clusters) affects their ability to displace surrounding water molecules and rotate through different symmetry axes, which correspondingly affects the rate at which protons can attack and melt them.
The details of all this hinge on, or are at least theorized to benefit from the particular qualities of the vesicle-based transporters installed on glutamatergic synapses. Originally discovered as sodium-dependant bit kidney phosphate transporters, sequence homology studies found similar transporters (VGLUTs) at the presynapse that act in a pH-dependant fashion to fill vesicles with glutamte. Fisher proposes that these VGLUTs have dual role first in taking up presumptive Posner molecules during brief exposures of the fusion pore during classical exocytosis, and then later operate much in reverse to express decomposed phosphorus into the larger presynaptic space. Thereafter, at least in briefly talking to Fisher, the potential exists to reform Posner clusters here, which can in turn re-melt with each generating 18 calcium ions to contribute to transmitter release.
VGLUTs themselves come in different forms and future work may help identify their respective roles. Other esoteric phenomena are undoubtedly also involved at a deep level in synaptic function, and one need not look to far abroad to find hint of them. For example, we previously proposed a potential mechanism where these pH-dependant glutamate transporters play a role in recently reported neural shock wave events in order to generalize the observation from acetylcholinergic synapses to other amino acid-based transmitter systems.
In the meantime, Fisher intends to re-explore the older Li isotope work and further refine the mechanisms and any potential shortfalls of the Posner conception. As alluded to above, electron spin itself is still a relatively obscure concept in biology save for a few niche revelations on things like chemical compasses or other radical pair-pair inspired biologics. Yet free radicals (unpaired electron spins) have a magnetic moment 1,000 or so times larger than that of a proton. Their presence alone could be a significant factor in things like phosphorus nuclear spin decoherence.
More information: Quantum Cognition: The possibility of processing with nuclear spins in the brain, arXiv:1508.05929 [q-bio.NC] arxiv.org/abs/1508.05929
Abstract
The possibility that quantum processing with nuclear spins might be operative in the brain is proposed and then explored. Phosphorus is identified as the unique biological element with a nuclear spin that can serve as a qubit for such putative quantum processing - a neural qubit - while the phosphate ion is the only possible qubit-transporter. We identify the "Posner molecule", Ca9(PO4)6, as the unique molecule that can protect the neural qubits on very long times and thereby serve as a (working) quantum-memory. A central requirement for quantum-processing is quantum entanglement. It is argued that the enzyme catalyzed chemical reaction which breaks a pyrophosphate ion into two phosphate ions can quantum entangle pairs of qubits. Posner molecules, formed by binding such phosphate pairs with extracellular calcium ions, will inherit the nuclear spin entanglement. A mechanism for transporting Posner molecules into presynaptic neurons during a ``kiss and run" exocytosis, which releases neurotransmitters into the synaptic cleft, is proposed. Quantum measurements can occur when a pair of Posner molecules chemically bind and subsequently melt, releasing a shower of intra-cellular calcium ions that can trigger further neurotransmitter release and enhance the probability of post-synaptic neuron firing. Multiple entangled Posner molecules, triggering non-local quantum correlations of neuron firing rates, would provide the key mechanism for neural quantum processing. Implications, both in vitro and in vivo, are briefly mentioned.
Journal information: arXiv
© 2015 Phys.org