Strengthening weak cohesive forces among molecules and atoms by light
Researchers led by Yoshiyuki Miyamoto predicted from numerical simulations that weak cohesive forces acting among molecules and atoms that are not chemically bonded can be strengthened by irradiation of light whose wavelength (related to its energy) is tuned. The cause of strengthening the cohesive forces is induction of oscillating positive and negative charges on molecules/atoms which generates electric attraction. The phenomenon predicted in the present research is expected to be applied to formation technology for structures built by the weak cohesive forces such as molecular crystals, which are used for organic devices.
Details of this research will be published in Applied Physics Letters, a scientific journal published by American Institute of Physics, on May 20, 2014.
Devices made of organic materials attract substantial attention because of their advantages such as lower production cost than that of semiconductors. Formation technology of molecular crystals with high quality is required for high device performance. Controlling inter-molecular interaction is thus a key technology for that purpose.
Cohesive force acts between molecules/atoms that are not chemically bonded. The force is called van der Waals force, which is popular as a source of adsorption power of geckos crawling on walls and as a source of inter-layer interaction of layered materials like graphite. The van der Waals force attracts high attention as cohesive force of molecular crystals used for organic devices. Optical tweezers manipulate molecules by light. However, it is difficult to apply force to individual molecules and atoms using optical tweezers.
AIST aims to develop technologies for constructing complex systems that consist of materials having well-controlled nano-scaled structures. By performing simulations based on the first-principles calculations, AIST proposed several fabrication technologies prior to practical experiments. Especially, a simulation scheme applied for fabrication using electronic excitation is a unique technology among research institutes in the world.
Current research was motivated to establish formation processes of molecular crystals by strengthening the van der Waals force with light. As the first step of the research, a simple object possessing the similar cohesive forces was selected to examine photo-induced strengthening of forces by simulations. To perform numerical computations needed for the simulations, the massive parallel computing system introduced into AIST, i.e. AIST-super cloud; Generation2, was used.
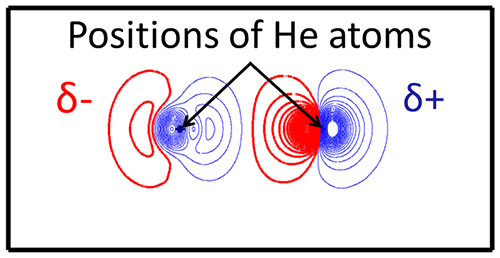
The researchers performed simulations of helium (He), which is the simplest chemically inert element. Electrons of He can change their orbital, i.e. electron excitation, with a certain amount of energy. When He is irradiated with light having photon energy (wavelength) very close to the excitation energy, electrons of He atoms start to oscillate to try to change their orbital, and the amplitude of the oscillation gradually grows with continuous irradiation. This is similar to the increase in the amplitude of a swinging pendulum with an external force having the same period of the swing. As shown in Fig. 1, at a moment when the amplitude of oscillation of electric charge distribution becomes maximum, positive and negative charges exist between two He atoms that cause attraction between the atoms, i.e. the dipole-dipole attraction. The electric charge distribution varies as time elapses, and positions of positive and negative charges are reversed at some moments, yet the charge distribution is always kept so as to make the two He atoms attract each other. Therefore, the dipole-dipole attraction is retained at all moments.
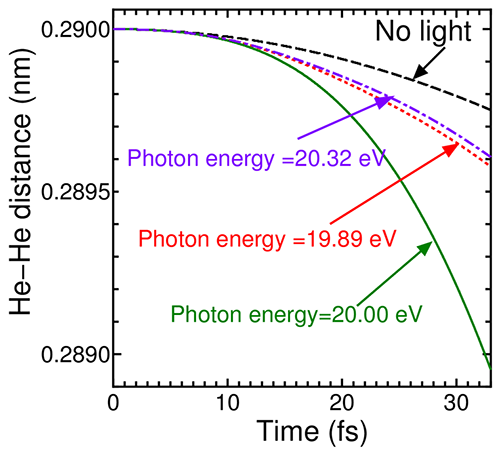
As displayed in Fig. 2, the distance between the two He atoms is significantly reduced by light irradiation compared to the case without light irradiation. This depicts sustained attractive force by light irradiation. Furthermore, the degree of reduction depends on the photon energy, and it is noted from this figure that light with photon energy of 20 eV gives the maximum attracting force.
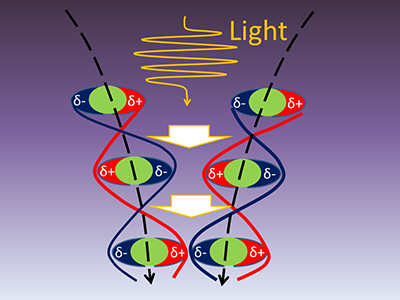
By analyzing time-evolution of the distance between the two He atoms, the attracting force was found to be increased by 7 pN compared to the case without light ("p" means one trillionth, 10-12). Although this quantity corresponds to a weak force, which is one thousandth of forces connecting atoms by ordinary chemical bonds, this is enough to manipulate atoms/molecules which are not chemically bonded. These fundamental results, obtained by simulation, also suggest photo-induced strengthening of cohesive forces between molecules in molecular crystals.
The researchers will examine more complex condensed systems for potential strengthening of van der Waals force by light, and will use the acquired knowledge to develop controlled fabrication technology of molecular crystals that can be used for devices.
Journal information: Applied Physics Letters
Provided by Advanced Industrial Science and Technology







.jpg)