April 25, 2011 feature
Chemists fabricate 'impossible' material
(PhysOrg.com) -- When atoms combine to form compounds, they must follow certain bonding and valence rules. For this reason, many compounds simply cannot exist. But there are some compounds that, although they follow the bonding and valence rules, still are thought to not exist because they have unstable structures. Scientists call these compounds "impossible compounds." Nevertheless, some of these impossible compounds have actually been fabricated (for example, single sheets of graphene were once considered impossible compounds). In a new study, scientists have synthesized another one of these impossible compounds -- periodic mesoporous hydridosilica -- which can transform into a photoluminescent material at high temperatures.
The researchers, led by Professor Geoffrey Ozin of the Chemistry Department at the University of Toronto, along with coauthors from institutions in Canada, China, Turkey, and Germany, have published their study in a recent issue of the Journal of the American Chemical Society.
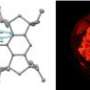
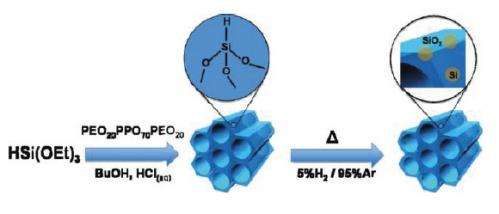
Like graphene, periodic mesoporous hydridosilica (meso-HSiO1.5) consists of a honeycomb-like lattice structure. Theoretically, the structure should be so thermodynamically unstable that the mesopores (the holes in the honeycomb) should immediately collapse into a denser form, HSiO1.5, upon the removal of the template on which the material was synthesized.
In their study, the researchers synthesized the mesoporous material on an aqueous acid-catalyzed template. When they removed the template, they discovered that the impossible material remains stable up to 300 °C. The researchers attribute the stability to hydrogen bonding effects and steric effects, the latter of which are related to the distance between atoms. Together, these effects contribute to the material’s mechanical stability by making the mesopores resistant to collapse upon removal of the template.
“The prevailing view for more than 50 years in the massive field of micro-, meso-, or macroporous materials is that a four-coordinate, three-connected open framework material (called disrupted frameworks) should be thermodynamically unstable with respect to collapse of the porosity and therefore should not exist,” Ozin told PhysOrg.com. “The discovery that this class of material can indeed exist with impressive stability is not a special effect related to the choice of the template, but rather that intrinsic hydrogen bonding between the silicon hydride O3SiH units and silanol O3SiOH that pervade the pore walls is strong enough to provide the meso-HSiO1.5 open-framework material with sufficient mechanical strength for it to be able to sustain the porosity intact in the as-synthesized template-containing and template-free material. This discovery is the big scientific surprise – so never say never when it comes to chemical synthesis.”
When raising the temperature above 300 °C, the researchers discovered that the mesoporous material undergoes a “metamorphic” transformation. This transformation eventually yields a silicon-silica nanocomposite material embedded with brightly photoluminescent silicon nanocrystals. Because the novel nanocomposite material retains its periodic mesoporous structure, the nanocrystals are evenly distributed throughout the structure. According to the researchers, the origin of the photoluminescence likely arises from quantum confinement effects inside the silicon nanocrystals.
In addition, the researchers found that they could control the photoluminescent properties of the nanocrystals by changing the thermal treatment. They predict that this ability could allow the bright nanocrystals to be used in the development of light-emitting devices, solar energy devices, and biological sensors.
“Now we have a periodic mesoporous hydridosilica in which we can exploit the chemistry of the silicon-hydride bonds that permeate the entire void space of the material,” Ozin said. “Every silicon in the structure has a Si-H bond to play creative synthetic games. This is a big deal in terms of it serving as a novel solid-state reactive host material within which one can perform novel chemistry limited only by one’s imagination, and a myriad new materials will emerge with a cornucopia of opportunities for creative discovery and invention.”
More information: Zhuoying Xie, et al. “Periodic Mesoporous Hydridosilica – Synthesis of an ‘Impossible’ Material and Its Thermal Transformation into Brightly Photoluminescent Periodic Mesoporous Nanocrystal Silicon-Silica Composite.” Journal of the American Chemical Society. DOI:10.1021/ja111495x
Copyright 2010 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.