Water most likely basis for complex ecosystem development

The assumption that alien biochemistries probably require liquid water may seem a little Earth-centric. But given the chemical possibilities available from the most abundant elements in the universe, even an alien scientist with a different biochemistry would probably agree that a water-solvent-based biochemistry is quite likely to occur elsewhere in the universe – and might well be the most likely foundation for a complex ecosystem in which intelligent life could develop.
Based on what we know of life and biochemistry, it seems likely that an alien biochemistry will need a solvent (like water) and one or more elemental units for its structure and function (like carbon). Solvents are important to enable chemical reactions, as well as physically transporting materials – and in both contexts, having that solvent in its liquid phase seems vital.
We might expect that common biochemically useful solvents are most likely to form from the most common elements in the universe – being hydrogen, helium, oxygen, neon, nitrogen, carbon, silicon, magnesium, iron and sulfur, in that order.
You can probably forget about helium and neon – both noble gases, they are largely chemically inert and only rarely form chemical compounds, none of which obviously have the properties of a solvent. Looking at what’s left, the polar solvents that might be most readily available in large volumes to support a biochemistry are firstly water (H2O), then ammonia (NH3) and hydrogen sulfide (H2S). Various non-polar solvents can also be formed, notably methane (CH4). Broadly speaking, polar solvents have a weak electric charge and can dissolve most things that are water-soluble, while non-polar solvents have no charge and act more like the industrial solvents we are familiar with on Earth, such as turpentine.
Isaac Asimov, who when not writing science fiction was a biochemist, proposed a hypothetical biochemistry where poly-lipids (essentially chains of fat molecules) could substitute for proteins in a methane (or other non-polar) solvent. It has been suggested that such a biochemistry could be supported on Titan.
Nonetheless, from the list of potentially abundant solvents in the universe, water looks to be the best candidate to support a complex ecosystem. After all, it is likely to be the most universally abundant solvent anyway – and it occurs in its liquid phase at a higher temperature range than any of the others.
It seems reasonable to assume that a biochemistry will be more dynamic in a warmer environment with more energy available to drive biochemical reactions. Such a dynamic environment is likely to mean that organisms can grow and reproduce (and hence evolve) that much faster.
Water also has the advantages of:
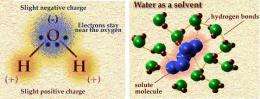
• having strong hydrogen bonds that gives it a strong surface tension (three times that of liquid ammonia) – which would encourage the aggregation of prebiotic compounds and perhaps the development of membranes;
• being able to form weak non-covalent bonds with other compounds – which, for example, supports the 3d structure of proteins in Earth biochemistry; and
• being able to engage in electron transport reactions (the key method of energy production in Earth biochemistry), by donating a hydrogen ion and its corresponding electron.
Hydrogen fluoride (HF) has been suggested as an alternative stable solvent that could also engage in electron transport reactions – with a liquid phase between -80 oC and 20 oC at 1 atmosphere pressure (Earth, sea-level). This is a warmer temperature range than the other solvents that are likely to be universally abundant, apart from water. However fluorine itself is not a very abundant element and HF, in the presence of water, will turn into hydrofluoric acid.
H2S can also be used for electron transport reactions – and is so used by some Earth-based chemosynthetic bacteria – but as a fluid it only exists in the relatively narrow and cold temperature range of -90 oC to -60 oC at 1 atmosphere.
These points at least make a strong case for liquid water being the most statistically likely basis for the development of complex ecosystems capable of supporting intelligent life. Although other biochemistries based on other solvents are quite possible – they seem likely to be limited to cold, low energy environments where the rate of development of biological diversity and evolution may be very slow.
The only exception to this rule might be high pressure environments which can sustain those other solvents in fluid phase at higher temperatures (where they would otherwise exist as a gas at a pressure of 1 atmosphere).
Provided by Universe Today