Carbon nanotube building blocks open up possibilities for advanced electronics
A new method to systematically modify the structure of single-walled carbon nanotubes could expand their electronic properties and open the path to nano-electronics.
Carbon cylinders a few billionths of a meter in diameter and a few microns long, these nanotubes are one of the strongest structures known and have unique electrical and thermal properties.
This promising method to add defects to carbon nanotube walls was developed by researchers at the U.S. Department of Energy's Argonne National Laboratory, who are interested in improving the materials for thermoelectric power generation, the use of heat differences to generate electricity. Thermoelectric conversion is the principle behind thermocouples, thermal diodes and solid-state refrigerators.
"If you change the electronic structure," said Argonne chemist Larry Curtiss, "by adding defects in an ordered way, theoretically you can make more efficient thermoelectric materials. So we could produce electricity more efficiently from solar, nuclear or any thermal power generation." Curtiss is group leader of the Molecular Materials Group in Argonne's Materials Science Division.
One dimer at a time
Creating defects by adding molecules to nanotubes is challenging because of their extremely small size. And researchers are seeking a controlled, reproducible method. So the Argonne team, which includes Curtiss, Michael Sternberg, Peter Zapol, Dieter Gruen, Gary Kedziora, Paul Redfern and David Horner, used computer simulation tools to learn how to add a single carbon dimer – a molecule of two bonded carbons – to a single-walled carbon nanotube.
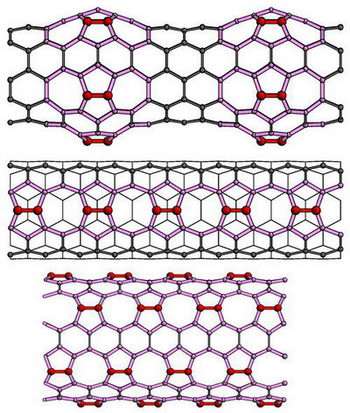
The single-walled nanotubes – believed to be the best candidates for next step of miniaturizing modern electronics – resemble a long tube of chain-link fence made of hexagons. The Argonne team simulated a variety of approaches to attach the carbon dimer to the nanotube. They found the easiest and strongest method is by horizontally inserting a carbon dimer into two hexagonal bonds, creating two adjacent pentagons and heptagons (seven-sided structures) in the chain link.
One dimer, two dimer…
After they understood how to add one dimer, the researchers began to add dimers in patterns.
"The interesting thing was going into the multiple patterns," Curtiss said. "We started building up patterns using the dimers like building blocks and adding them to the tubes."
The researchers found a number of interesting modifications:
-- The "bumpy" tube has carbon dimers added symmetrically around the circumference of the tube to create a stable bulge.
-- The "zipper" tube has dimers added horizontally along the axial direction to every other hexagon, creating alternating single octagons and pairs of pentagons.
-- The "multiple zipper" tube has six axial "zippers" spaced by hexagon rows around a tube.
"The structures we simulated," said physicist Zapol, "have new and unexpected features. They modify the electronic properties in the nanotubes, and that will be useful in future electronic applications."
Guided by the simulations, Argonne materials scientists, led by Gruen, with expertise in carbon nanomaterials are creating materials for testing.
"But we think that some of these structures exist already," said Curtiss. Zapol's literature review revealed that some researchers have found these structures, but they did not know what they were.
The zipper structure particularly appeals to Argonne researchers because the atomic spacings in the openings are just the right size to bond nanotubes to Ultrananocrystalline™ diamond and combine the properties of both.
Ultrananocrystalline diamond is a novel form of nanocarbon developed by Argonne that has many of the properties of diamond – the hardest known material on earth – and can be deposited on a variety of surfaces. Unlike diamond, its properties can be optimized depending on the application.
Researchers plan to use the carbon nanotubes as a scaffolding to attach other molecules and study their functions. They will also connect the tubes into arrays and study the effects.
Source: by Evelyn Brown, Argonne National Laboratory





















