Efficient method for photocatalytic fluoroalkylations of (hetero)arenes
Fluoroalkylated aromatic compounds have properties such as permeability, lipophilicity and metabolic stability. Considerable efforts have been devoted to the development of efficient methods for incorporation of the fluoroalkyl group into aromatic frameworks. However, many commonly used fluoroalkylating reagents are expensive or operationally inconvenient, and some of them require multistep syntheses.
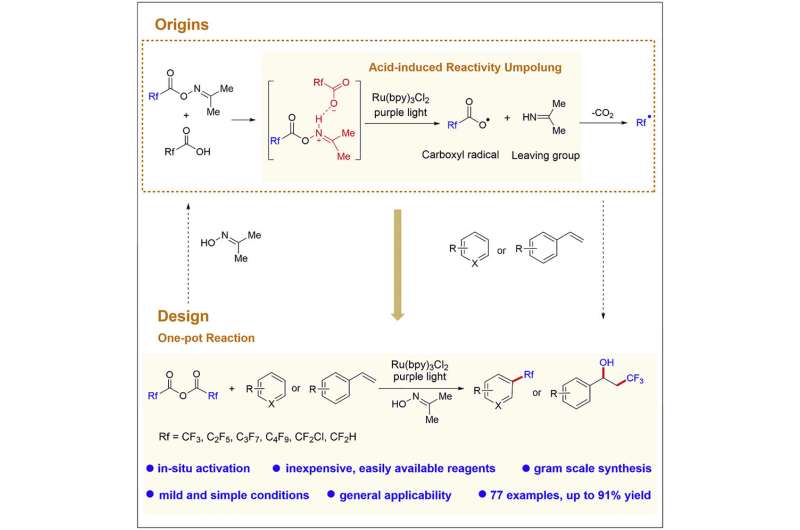
Recently, a research group led by Prof. Su Weiping from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences reported a general photocatalytic method for regioselective C-H fluoroalkylation of (hetero)arenes using readily available fluoroalkyl carboxylic anhydrides as fluoroalkylating reagents in the presence of simple acetoxime as an activator.
The study was published in Chem Catalysis on June 16.
The C-H fluoroalkylation method is attributed to the discovery of the acid-triggered reactivity umpolung of acetoxime ester towards single-electron reduction-induced cleavage of the N-O σ-bond.
The researchers evaluated the feasibility for the photocatalytic reduction-triggered decarboxylation of acetoxime trifluoroacetate to generate CF3 radical. Then, they developed an in-situ activation strategy for photocatalytic trifluoromethylation of (hetero)arenes via in-situ generation of acetoxime trifluoroacetate and TFA from trifluoroacetic anhydride (TFAA) and acetoxime.
The mechanistic studies demonstrated that the interaction between TFA and acetoxime ester resulted in the photoredox-mediated cleavage of N-O σ-bond of acetoxime ester to generate trifuoromethyl carboxyl radical and imine. This was opposite to the inherent reactivity of N-O σ-bond of acetoxime ester toward the generation of an iminyl radical and trifluoroacetate anion under photoredox catalysis, demonstrating unprecedented acid-triggered reactivity umpolung of acetoxime ester.
The activator acetoxime used in this study is inexpensive, nontoxic, stable to air and simple to handle. This acetoxime/fluoroalkyl carboxylic anhydrides combination has a low cost and high in-situ activation efficiency. It can avoid cumbersome presynthesis and the resulting byproducts, 2-propanimine or acetone, are small, volatile and easily removed.
This study not only provides an efficient approach to the fluoroalkylation of (hetero)arenes and alkenes, but also discloses a novel reactivity mode for photo-driven cleavage of σ-N-O bond in oxime-esters, which will help to expand the synthetic application of readily available oxime-esters.
More information: Min Zhang et al, Photocatalytic fluoroalkylations of (hetero)arenes enabled by the acid-triggered reactivity umpolung of acetoxime esters, Chem Catalysis (2022). DOI: 10.1016/j.checat.2022.05.018
Provided by Chinese Academy of Sciences