October 1, 2019 report
Chemists synthesize perseanol for the first time
![a, Chemical structure, carbon numbering and ring system letter assignment for the ryanodane (a) and isoryanodane (b) diterpenes. c, Retrosynthetic analysis of the isoryanodane diterpene (+)-perseanol. [O], oxidation; R, protecting group. Credit: Nature (2019). DOI: 10.1038/s41586-019-1580-x Chemists synthesize perseanol for the first time](https://scx1.b-cdn.net/csz/news/800a/2019/1-chemistssynt.jpg)
A team of chemists at California Institute of Technology has totally synthesized perseanol using a 16-step process for the first time. In their paper published in the journal Nature, the group describes their process and how well it worked.
In nature, perseanol is a molecule produced by the persea tree. Shortly after its discovery in the late 1990s, researchers found that the molecule was similar to ryanodine, a once-popular pesticide. They have similar architecture, though perseanol lacks a pyrrole-2-carboxylate ester. Because of the similarities, interest in using perseanol on commercial crops grew. Not much later, a cheaper alternative was found, and the molecule never made it to the farm. But interest in it persists because of its ecofriendly reputation. For that reason, chemists have been working to produce it in the lab—if successful, the results would be both cheaper and more environmentally friendly than products now in use.
The researchers note that ryanodine works as a pesticide by binding to calcium channels in insects' muscles, paralyzing them. It can paralyze animals, too, but perseanol is believed to be more specific to insects, making it a potentially safer pesticide. The researchers also note that little research has been performed to determine the means by which perseanol kills bugs. That could change however, if interest in using perseanol as a pesticide is rekindled.
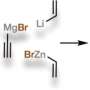
The researchers started with lessons they learned when they synthesized ryanodine two years ago. To do the same with perseanol, the team started with an oil called pulegone and a vinylogous ester. Fragments of each were modified using a six-step process and were then brought together using a cascading technique, which resulted in building the structure of the molecule. Eight more steps were required to add side chains.
The researchers note that the complexity of the molecule makes it unlikely that it would be used as an agrochemical, but their work has led to a better understanding of the family of molecules that perseanol belongs to. This could lead to the development of other products. It also opens the door to better understanding how it kills insects and how it compares to ryanodine.
More information: Arthur Han et al. A 16-step synthesis of the isoryanodane diterpene (+)-perseanol, Nature (2019). DOI: 10.1038/s41586-019-1580-x
Journal information: Nature
© 2019 Science X Network