Salk scientists adapt powerful gene-editing system to correct dementia in lab
The revolutionary CRISPR/Cas9 gene editing system made it possible to rapidly and precisely alter DNA, the essential molecule of life. But DNA doesn't work by itself, it relies on the messenger molecule RNA to carry out its instructions.
Salk Institute scientists reported recently that they've invented a new version of the technology that works on RNA, combining CRISPR/Cas9's precision with the ability to turn its effects on and off at will. And because it leaves DNA untouched, it's safer.
A form of dementia may eventually be treatable with the technology, called CasRx. But much more work needs to be done before it can be tried in patients.
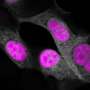
Working in cultures of brain cells, the scientists corrected a protein imbalance that causes frontotemporal dementia with parkinsonism. They are preparing to test CasRx in live animals, said Patrick Hsu, a Salk researcher and study leader.
If that work goes well, the method could be tried in patients. Moreover, CasRx could become a general platform technology for treating genetic diseases, the study said.
The study was published in the journal Cell.
The scientists packaged a custom-designed enzyme into a virus often used to deliver genes to DNA. But instead of changing DNA like CRISPR/Cas9, the enzyme altered the function of a messenger molecule called RNA, which carries out DNA's instructions. (The Cas9 refers to the enzyme used. CasRx employs enzymes from the Cas13 family).
CRISPR/Cas9 acts like molecular scissors on DNA. Because it is fast and precise, the technology has been likened to the impact of a word processor on writing. A mutation that causes a genetic disease can be simply edited out or corrected, in a cell, organs, and eventually people.
But CRISPR/Cas9 isn't totally accurate, and any mistake could cause permanent damage to the genome. This risk has caused scientists to be cautious about developing it for therapy.
By targeting RNA, the Salk Institute scientists got around this problem.
Because the new CRISPR method leaves the DNA intact, its effects aren't permanent, Hsu said. RNA is continually generated. So withdrawing the treatment allows the RNA function to return to its previous state.
Accuracy is another advantage, the scientists said.
Many other methods of changing RNA function for therapeutic uses already exist. One of the most popular is called RNA interference, or RNAi. The study found that the new CRISPR method works better than RNAi.
Any improvement in accuracy is vital for RNA therapy, said Floyd Romesberg, a prominent scientist at The Scripps Research Institute.
"If you're getting what you want in 98 percent of cells, and in 2 percent of the cells you're doing something else, that's not good," said Romesberg, who was not involved in the study. "That could cause cancer, that could do all sorts of things."
Perhaps even more important is the novel method the scientists used to discover this new family of CRISPR, Romesberg said. Their approach could unlock a trove of new genetic engineering tools.
CRISPR systems originated in nature as a bacterial immune system. They contain two main components, a genetic sequence matched to a known pathogen, called CRISPR, and an enzyme that cuts up genetic material containing the sequence. The enzyme most used is called Cas9, hence CRISPR/Cas9.
Given the profusion of bacterial species, it stands to reason that a plethora of CRISPR systems are waiting to be discovered, Romesberg said.
More information: Silvana Konermann et al. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors, Cell (2018). DOI: 10.1016/j.cell.2018.02.033
Journal information: Cell
©2018 The San Diego Union-Tribune
Distributed by Tribune Content Agency, LLC.