Inflammation awakens sleepers
The inflammatory response that is supposed to ward off pathogens that cause intestinal disease makes this even worse. This is because special viruses integrate their genome into Salmonella, which further strengthens the pathogen.
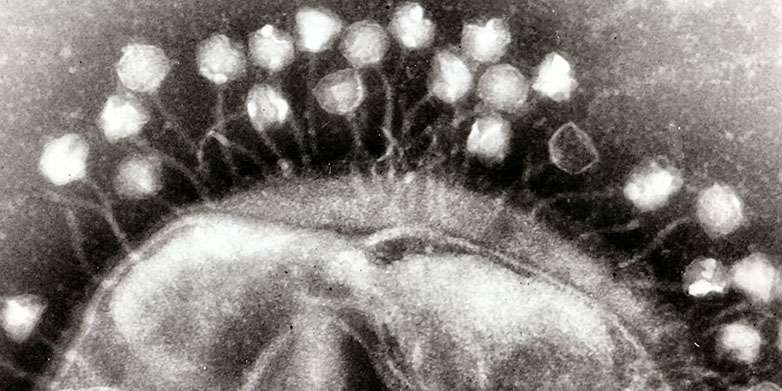
Bacteriophages (short form: phages) are viruses that infect bacteria. The "good" lytic phages kill off bacteria that are harmful to humans and are sometimes used in medicine; the "bad guys", the temperate phages, on the other hand, transfer their genes to microorganisms, thus giving them new properties, such as the ability to produce a toxin. The transfer of temperate phages is therefore regarded as an important driving force behind the development of bacteria into potent pathogens (see box).
Using Salmonella, a common pathogen of gastrointestinal diseases, as an example, researchers led by ETH Professor Wolf-Dietrich Hardt have now shown that the body's own inflammatory response actually promotes the transfer of phage genes to the bacteria, thus increasing the pathogenicity of Salmonella. Their study has just been published in the specialist journal Science.
Highly efficient gene transfer
To find out how quickly temperate phages spread out within a Salmonella population, the researchers infected mice with two different strains of Salmonella. One strain carried the "SopEΦ" phage, while the other did not.
Salmonella triggered an inflammation in the animals' intestine, which led to a major change in the Salmonella strain carrying phage genes: the phage genes were expressed, the phage multiplied and ultimately free phage particles were released, killing the Salmonella cell. Free phages swarmed out and entered the second Salmonella strain to further increase there. In this way, the phages transferred their genes to almost all Salmonella cells of any strain that had previously been free of phage genes.
This horizontal gene transfer sometimes took only three days to complete. "The gene transfer is extremely efficient. That surprised us," says Hardt, who had not expected such a rapid spread of infection into naive Salmonella strains.
Virus is linked to an alarm system
"The efficiency of the procedure can be explained using previous knowledge gained from textbooks," says Médéric Diard, a postdoc in Hardt's group, who carried out the study. As soon as the bacteria cell is attacked by inflammatory factors such as reactive oxygen and nitrogen species, it sets off an SOS signal, which starts the cell's own repair programme. This signal is used as a wake-up by the phages lying dormant in the genome. "Our results show that the inflammation of the intestine promotes the horizontal gene transfer through phages – an important evolutionary mechanism of microorganisms," explains Hardt.
As long as the inflammation persists, the freshly infected Salmonella also produce more phages that in turn infect more Salmonella. This chain reaction can only be prevented if the adaptive immune system intervenes. It sends specific antibodies to neutralize Salmonella at the site of infection.
This risk of phage release can be alleviated by vaccination: Salmonella in vaccinated animals is prevented from triggering a bowel inflammation. Incidentally, this also prevents the SOS response and the production of phages.
Virus as a beneficiary
Médéric Diard proposed that phages could take "control" of the bacteria so that they trigger inflammation ever more efficiently. This also promotes phage reproduction n the intestine. This link may explain why many phages transfer toxin genes to the bacteria. Phage-encoded toxic substances may create the very conditions in the intestine of victims that stimulate phage production. "Phages are 'selfish'. Therefore one may regard Salmonella diarrhea as a collateral damage of phage evolution," says Hardt.
How the cholera bacterium became viciously successful
Cholera has become a dreadful cause of diarrhea worldwide. claimed many lifes. This was not always the case. The original ancestor of Vibrio cholerae was a harmless brackish water bacterium off the coast of Bangladesh. A phage infected these bacteria and integrated into their genome, including the cholera toxin. This turned the harmless bacterium into a powerful pathogen. The acquisition of the cholera toxin gene obviously provides this bacterium with an evolutionary advantage. Today, the bacterium has spread around the world and frequently causes epidemics, claiming many lifes, particularly after natural catastrophes or in areas of conflict where there is poor hygiene.
More information: Médéric Diard et al. Inflammation boosts bacteriophage transfer betweenspp., Science (2017). DOI: 10.1126/science.aaf8451
Journal information: Science
Provided by ETH Zurich