Underappreciated protein plays critical role in RNA regulation and male fertility
A protein once thought to be of little consequence has been found to be a central player in processes ranging from male fertility to early embryonic development, according to a study published in the March 31 online issue of Cell by researchers at the University of California, San Diego School of Medicine.
"We've obtained several lines of evidence that the UPF3A protein is a potent suppressor of the nonsense-mediated RNA decay (NMD) pathway," said Miles Wilkinson, PhD, senior author and professor in the Department of Reproductive Medicine at UC San Diego School of Medicine. "UPF3A was previously thought to be just the opposite—a promoter of the NMD pathway, but a weak one that had little effect. Thus, UPF3A was largely ignored by the field."
The NMD pathway is a critical quality control mechanism used by cells to eliminate faulty messenger RNAs (mRNAs) - the molecules responsible for transmitting genetic code. If not degraded, these aberrant mRNAs lead to the formation of short versions of proteins that can raise havoc in cells. "By preventing the production of these truncated proteins, NMD is thought to protect against many diseases, including cancer, diabetes and a wide variety of genetic diseases," said Wilkinson.
To increase the effectiveness of the NMD pathway, Wilkinson said, drugs could be designed to inhibit UPF3A, as it is a natural suppressor of the pathway. Diseases that could potentially be treated include diabetes, amyotrophic lateral sclerosis and Marfan syndrome, a genetic disease of the connective tissue.
"Since 15 to 30 percent of all human genetic diseases are caused by mutations detected by NMD, the range of genetic diseases potentially treatable by 'NMD therapy' is vast," said Samantha Jones, a PhD student in Wilkinson's lab who is co-first author on the study.
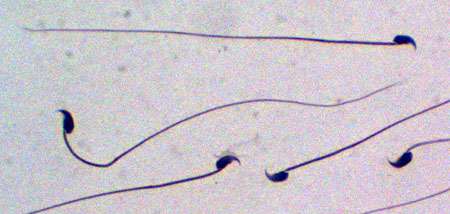
Because UPF3A is highly expressed in male testes, the researchers also explored whether UPF3A might have a role in fertility. In mouse experiments, they knocked out UPF3A in male germ cells—the cells that give rise to sperm—and found these mutant mice generated few sperm.
The researchers found that loss of UPF3A greatly reduced the number of cells in the testes that undergo meiosis, a process unique to the cells that give rise to sperm and eggs. "While more study is needed, it is possible that loss of UPF3A causes a defect in the meiosis process itself," said Wilkinson.
The research team also globally knocked out UPF3A in mouse models and found this led to early embryonic death. "This suggests that the NMD inhibitory function of UPF3A is also crucial in the very first stages of development in embryos," said Jones.
"Another twist to this story is that UPF3A has a sister protein called UPF3B, which is encoded by the X chromosome and is critical for normal human cognition," said Wilkinson. Its loss in humans causes intellectual disability and is highly linked to neurodevelopmental disorders, like autism and schizophrenia.
Unlike UPF3A, UPF3B is an activator of the NMD pathway. Wilkinson said this has evolutionary implications since the two genes that give rise to these two proteins come from a single gene that duplicated approximately 500 million years ago, when the vertebrates first appeared.
"While it is well accepted that gene duplication drives the generation and adaptation of species, it remains a poorly understood evolutionary force," Wilkinson said. Particularly mysterious has been what prevents newly duplicated genes from deteriorating. Wilkinson and colleagues discovered a simple mechanism that likely allowed the duplicated UPF3A gene to avoid decay 500 million years ago. It lost sequences essential for activity and became a repressor. "At a genetic level, it's a lot easier to lose something than gain something."
The finding that UPF3A and UPF3B have opposite effects suggests they are the product of a relatively rare evolutionary outcome of gene duplication—functional antagonism. UPF3B turns NMD "on" while UPF3A turns NMD "off."
But why does NMD have such an "on" and "off" switch? A likely answer comes from the well-established finding that NMD is not only a quality control mechanism, but also a pathway that degrades many normal mRNAs. "By degrading batteries of mRNAs encoding proteins at specific points in development, NMD can potentially greatly influence development," said Wilkinson.
Indeed, many laboratories have found that NMD is critical for the development of several cell types in organisms ranging from flies to humans. "Our results suggest that UPF3A and UPF3B act as volume controls to up- and down-regulate NMD at the right times for normal development to proceed," Wilkinson said.
More information: Cell , dx.doi.org/10.1016/j.cell.2016.02.046
Journal information: Cell
Provided by University of California - San Diego