Shaking the electron has strengthened quantum mechanics
Decays of atomic nuclei are potential sources of information on fundamental phenomena occurring in the quantum world. Unfortunately, it is a rather difficult task to model such processes. However, NCBJ physicists have successfully simulated the process of neutron-proton conversion in a singly ionized 6He atom nucleus, and correctly predicted its impact on the atomic orbital sole electron. Theoretical calculations were recently confirmed by an experiment performed in the GAEN accelerator centre in Caen (France).
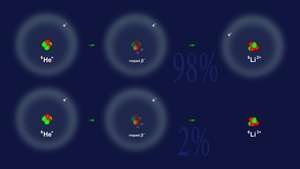
Nucleus of a 6He ion is composed of two protons and four neutrons. In a singly ionized ion the nucleus is orbited by a single electron. Surplus of neutrons makes such nuclei unstable—they undergo the so-called beta-minus decays in which one of the neutrons is transformed into a proton. To preserve electric charge, an electron is emitted from the decaying nucleus. Each emitted electron is accompanied by an electron anti-neutrino. In effect, a stable 6Li nucleus (still orbited by a single electron) is produced.
"During each beta-minus decay, the orbiting (atomic) electron is impacted because of two reasons. Firstly, the total electric charge of the nucleus is changing since three protons are suddenly appearing in place of two protons. Secondly, a negatively charged emitted electron is flying nearby. It is plenty of stimulation for the orbiting electron: One might say that it is shaken very strongly. As a result, it is excited to a higher orbital or completely struck out of the atom," explains Professor Zygmunt Patyk from NCBJ.
Quantum mechanics uses wave functions to describe particles. The functions may be used to calculate probabilities that the particle will take some determined states.
"The 6He ion selected for calculations is almost a textbook case: single electron orbiting within a relatively simple potential well of the nucleus," said Dr. Katarzyna Siegień-Iwaniuk from NCBJ.
Electrons emitted by the decaying nuclei move at a speed close to the light velocity. They cross orbital electron clouds in times shorter than one billionth part of a nanosecond (i.e. 10-18 s). In quantum mechanics, problems with such short interactions are treated by finding a superposition of some final state wave functions (in our case: single electron within the 6Li ion) that jointly approximate the initial state wave function (in our case: single electron within the 6He ion). This trick is known as the sudden approximation method. It has been applied for many years—almost from the days quantum mechanics was born—but has never been directly verified in any experiment.
Professor Patyk's team has been collaborating with teams of physicists working at GAEN accelerator centre in Caen (Normandie, France) for several years. Calculations performed by NCBJ physicists to the accuracy of four significant places yielded the 2.3% probability that beta-decay will be liberating the sole orbital electron of the 6He ion, i.e. will be producing a totally ionized lithium atom. To a comparable accuracy, that result was confirmed by some experiments performed at the French accelerator.
"Such a good agreement between theoretical predictions and experimental findings in such a simple (almost textbook) system is the first direct proof that the sudden approximation computational method utilized to solve quantum mechanics problems for almost a century is indeed correct," points out Professor Patyk.
NCBJ physicists have also managed to determine factors responsible for liberating the 6He sole orbital electron. The performed analyses have indicated that the ionization is caused in 99% cases by change of the nucleus total electric charge, and only in 1% cases by the fast electron emitted by the decaying nucleus.
Provided by Narodowe Centrum Badan Jadrowych