Study on effect of electricity on liquids bucks conventional science (w/ Video)

(PhysOrg.com) -- Whether gazing into lava lamps or watching balsamic vinegar mix with olive oil, people have long been transfixed by the seemingly mystical way that droplets of one liquid find each other within another liquid and join together. Conventional scientific wisdom has held that this merging of liquid droplets, a process called coalescence, is enhanced by applying an electrical field, but a new study, which will be published in the Sept. 17 issue of the journal Nature, shows that an increased electrical field actually can prevent droplets from merging.
"These surprising results could lead to improved applications in diverse fields including petroleum purification, food-oil processing and even biodiesel production," said Andrew Belmonte, an associate professor of mathematics at Penn State and one of the leaders of the project. "The results also could increase our understanding of atmospheric high-voltages that are generated in thunderstorms."
According to William Ristenpart, an assistant professor at the University of California at Davis and another of the project's leaders, "It has long been assumed that oppositely charged droplets experience an attractive force that encourages them to coalesce. This study, however, demonstrates that while droplets move toward each other when a low-strength electrical field is applied, those same droplets actually are repelled from one another after they make contact under higher-strength electrical fields."
Belmonte explained the process with a household example.
"Say you have a bottle of salad dressing -- vegetable oil with droplets of vinegar. If you put two electrodes into the dressing, you would produce an electric field, and the droplets of oil and vinegar may become either positively or negatively charged. We used to think that oppositely charged droplets would be attracted to each other and coalesce, and that the electric field would bring them together even faster. Now we know that if the electric field -- or voltage -- is high enough, oppositely charged droplets will not coalesce. Instead, when they come into contact with one another, they bounce and move in opposite directions."
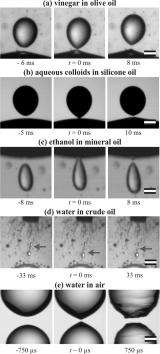
In their experiment, the research team observed that water droplets, sitting in oil between two electrodes, appeared to bounce off each other under strong electrical fields. High-speed video and still images revealed that, between bouncing, a temporary fluid "bridge" formed between the meniscus, or curved surface, of each droplet. That temporary bridge, which existed for less than 40 microseconds before it snapped, seems to have been responsible for transferring an electrical charge between the droplets.
"It appears that the charge from the electrode at the top of the cylinder caused one droplet to fall through the oil until it momentarily made contact with another droplet, and then the falling droplet quickly bounced back up," Ristenpart said. "The fact that the top droplet was able to move upward, against the force of gravity, suggests there was a change in the net electrical charge of the droplet and that the charge was transmitted during the bounce."
According to Belmonte, the surface tension naturally occurring between oil and water makes droplets spherical.
"We know that electric fields can elongate a single water droplet, leading eventually to a sharp point known as a Taylor cone," he said. "We see something like these cones just before the two droplets merge, a fleeting shape resembling an elongated hour glass."
The researchers propose that when the electrical field is strong enough, the hour glass becomes too "steep" to hold up against surface tension. As a result, the bridge breaks, but not before allowing the electrical charge to be transmitted between the droplets.
Belmonte, who performed the experiment while he was on sabbatical leave from Penn State at Harvard University, plans to rebuild the device at Penn State in the W.G. Pritchard Laboratories in order to further study the phenomenon.
"Among other things, we're interested in investigating the chemical reactions that could be placed inside the droplets," he said.
Provided by Pennsylvania State University (news : web)