Findings Could Improve Fuel Cell Efficiency
Researchers at Duke’s Pratt School of Engineering have developed a membrane that allows fuel cells to operate at low humidity and theoretically at higher temperatures.
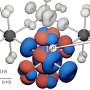
A new type of membrane based on tiny iron particles appears to address one of the major limitations exhibited by current power-generating fuel cell technology.
While there are many types of fuel cells, in general they generate electricity as the result of chemical reactions between an external fuel -- most commonly hydrogen -- and an agent that reacts with it. The membrane that separates the two parts of the cell and facilitates the reaction is a key factor in determining the efficiency of the cell.
Fuel cells are commonly used in such settings as satellites, submarines or remote weather stations because they have no moving parts, do not require combustion and can run unattended for long periods of time. However, current fuel cells lose efficiency as the temperature rises and the humidity falls.
Researchers at Duke University’s Pratt School of Engineering have developed a membrane that allows fuel cells to operate at low humidity and theoretically to operate at higher temperatures. They reported their findings online in the Journal of Membrane Science.
“The current gold standard membrane is a polymer that needs to be in a humid environment in order to function efficiently,” said Mark Wiesner, Ph.D., a Duke civil engineering professor and senior author of the paper. “If the polymer membrane dries out, its efficiency drops. We developed a ceramic membrane made of iron nanoparticles that works at much lower humidities. And because it is a ceramic, it should also tolerate higher temperatures.
“If the next series of tests proves that fuel cells with these new membranes perform well at high temperatures, we believe it might attract the type of investment needed to bring this technology to the market,” Wiesner added.
The membrane most commonly used today, known as Nafion, was discovered in the 1960s. As the temperature rises, the polymer becomes unstable and the membranes dehydrate, leading to a loss of performance.
In addition to its temperature and heat limitations, Nafion is also much more expensive to produce than the new membrane, Wiesner said, adding that membranes make up as much as 40 percent of the overall cost of fuel cells.
Wiesner said he believes that future experiments will demonstrate the new membrane’s ability to operate at higher temperatures.
“The efficiency of current membranes drops significantly at temperatures over 190 degrees Fahrenheit,” he explained. “However, the chemical reactions that create the electricity are more efficient at high temperatures, so it would be a big improvement for fuel cell technology to make this advance.”
An interesting outcome of these experiments is leading Wiesner down a new and related research path. As a result of the chemical reactions that create the electricity, small amounts of water are created as a byproduct.
“In the current technology, this water is used by the system to maintain the humidity within the cell,” Wiesner said. “The water produced in these reactions is of high purity. So, if a fuel cell membrane could be developed that wasn’t reliant on humidity, this water could be used for other purposes.”
In addition to these experiments, Wiesner’s team plans to study new ways of fabricating the membranes to improve their durability and flexibility.
The study is available here.
Source: Duke University