Oscillating Pattern in Nanoparticle Crystallisation
In order to survive, biological systems need to form patterns and organise themselves. Scientists at the Max Planck Institute for Colloids and Interfaces in Potsdam, Germany, have now combined self-organisation with chemical pattern formation. They coupled an oscillating chemical reaction with polymer-controlled crystallisation and self-organisation in barium carbonate.
In this way, they proved that oscillating reactions - like the renowned Belousov-Zhabotinsky reaction - can also take place in multi-phase systems. On basis of these results, scientists can better explain chemical reactions which are out of thermodynamic balance, as well as biological pattern formation in nature. Furthermore, these results could lead to the creation of surfaces with new kinds of structures.
Scientists are especially interested in oscillating chemical reactions. These occur when reaction products periodically and repeatedly change. Their behaviour is of importance to many fields of study - including chaos research. That is because these reaction systems are always complex and far away from thermodynamic equilibrium. One particularly well-known example is the "Belousov-Zhabotinsky" reaction. In it, a coloured indicator is used to make the reaction products of a coupled redox reaction visible. They typically take on the pattern of concentric circles, spreading out, for example, across a petri dish.
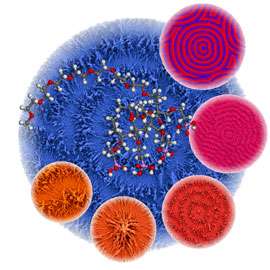
Mathematically, spatially oscillating reactions can be described as "reaction-diffusion systems". This means that it is not just chemical reactions which influence the amount of material at a certain point in space. Diffusion also plays a role - the exchange of material with the surrounding area. In such simulations, we get the typical concentric circle pattern of a Belousov-Zhabotinsky reaction. In the picture above, it is indicated in red-violet.
Researchers from Potsdam have now proven that these oscillating reactions can also apply to multi-phase systems, and even to the self-organisation processes of nanoparticles. What is central is that in a multi-phase reaction system, it is possible to formulate either an autocatalyic or autoinhibiting reaction step. This leads an oscillating system to be constructed, and ultimately a pattern to be formed.
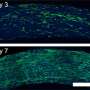
The researchers used a newly synthesized polymer to create the typical concentric circle pattern, via controlled barium carbonate crystallisation (see image). Such patterns correspond quite well to the calculations in a simulation. The researchers also were able to formulate a complex coupled reaction system including crystallisation, complexation, and precipitation reactions and identify the autocatalytic formation of a complex between barium and the polymer.
Notably, the elongated crystalline structures which made up the circular pattern are themselves created by superstructures of nanoparticles, which are themselves created by self-organisation (see image). In this way, Max Planck researchers have shown for the first time that the Belousov-Zhabotinsky reaction does not just take place in a solution, but also in multi-phase systems, and in nanoparticle self-organisation. This discovery is not only important to research into reactions far away from thermodynamic equilibrium. It can also help explain biological pattern formation. One example of biological self-organisation is mussel shell patterns. They are created via controlled crystallisation, just like the model systems of the researchers in Potsdam used. Interestingly, these patterns also mathematically duplicate reaction-diffusion systems exactly.
Citation: Tongxin Wang, An-Wu Xu, Helmut Cölfen, Formation of self-organized, dynamic structure patterns of barium carbonate crystals in polymer controlled crystallization, Angewandte Chemie (Online-Edition), June 21, 2006
Source: Max Planck Institute of Colloids and Interfaces