Serpentine locks up Carbon Dioxide

A common mineral can remove carbon dioxide from combustion gases, but in its natural state, it is glacially slow. Now, a team of Penn State researchers is changing serpentine so that it sequesters the carbon dioxide from fossil fuel burning in hours, not eons.
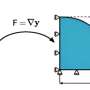
"Serpentine naturally sequesters carbon dioxide over geologic time, but it is too slow to help us," says Dr. M. Mercedes Maroto-Valer, assistant professor of energy and geo-environmental engineering and program coordinator for sustainable energy, the Energy Institute.
The metamorphic mineral serpentine -- or magnesium silicate hydroxide -- is composed of magnesium, silicon and oxygen and is plentiful. He researchers used material from the Cedar Hills quarry on the Pennsylvania/ Maryland border for this study, but the mineral is available in large quantities in many places. The U.S. deposits of the minerals that can be used for this process – serpentine and ovivine – can sequester all the carbon dioxide emissions produced from fossil fuels.
"Previous researchers investigating serpentine for use in sequestering carbon dioxide have crushed serpentine very finely, to sizes smaller than beach sand, but, even at these small sizes, it takes high temperatures to speed up the reaction, "says Maroto-Valer. "With our method, we do not need to crush it that fine and we do not need high temperatures. In fact, the reaction gives off heat. Our method is much less energy expensive."
The researchers, who also include John M. Andresen, director of the Consortium for Premium Carbon Products from Coal (CPCPC), the Energy Institute; Yinzhi Zhang, post doctoral fellow, the Energy Institute; Matthew E. Kuchta, graduate student in geo-environmental engineering, all at Penn State; and Dan J. Fauth, U.S. Department of Energy’s National Energy Laboratory in Pittsburgh, dissolved the crushed serpentine in sulfuric acid.
When serpentine dissolves in sulfuric acid, the silicon in the mineral becomes silicon dioxide, or sand, and falls to the bottom, while the magnesium becomes magnesium sulfate. Treating some of this magnesium sulfate with sodium hydroxide also creates some magnesium hydroxide. The researchers were able to convert large amounts of the serpentine’s magnesium to these chemicals providing large surface areas for reactions to occur in solution at room temperature.
Carbon dioxide passed through the solution of magnesium sulfate and magnesium hydroxide converts both to magnesium carbonate or magnesite, which becomes a solid and falls to the bottom. This solid can be used to manufacture construction blocks and there is also a small market for hydrated magnesium carbonate in the cosmetics industry. The silicon dioxide can be used to remove sulfur dioxide from the flue gases, which can subsequently be converted to sulfuric acid to use in the first part of the process.
"The high surface area of the silicon dioxide makes it a natural sorbent for capturing more carbon dioxide and sulfur dioxide," says Maroto-Valer.
The researchers have not yet tested the process on a working coal-fired stationary boiler, but they are working on developing a reactor in the laboratory that can continuously treat the flue gas. At the same time they would like to regenerate the sulfuric acid to minimize costs.
Because carbon dioxide will be the last gas in the emission stream treated, there are two options for commercial implementation. Fossil fuel burning plants could simply place a serpentine reactor as the last component of their emissions clean up and sequester carbon on site. Or, if the area is heavy with fossil fuel burning plants, each plant could pipe their carbon dioxide to a central treatment plant.
The U.S. Department of Energy supported this research. The researchers have applied for a U.S. patent for this process.