Polymeric nitrogen synthesised
The new single-bonded nitrogen phase could serve as a high-energy storage material, report Max Planck researchers in Mainz, Germany
Nitrogen, the major constituent of air, usually consists of inert molecules where two atoms are strongly triple-bonded. Now, researchers of the Max Planck Institute for Chemistry have synthesised a polymeric cubic form of nitrogen where all atoms are connected with single covalent bonds, similar to carbon atoms in diamond. This cubic phase has not been observed previously in any element. It possesses unique properties such as energy capacity: more than five times that of the most powerful explosives (Nature Materials, August 2004, published online 4 July 2004).
Single-bonded nitrogen was postulated theoretically two decades ago. It was predicted that at high pressure, solid molecular nitrogen would transform to an atomic solid with a single-bonded cubic gauche (cg-N) structure. There have been extensive experimental searches for this polymeric form of nitrogen at high pressures and various temperature ranges. Several new nitrogen phases have been found, including a non-molecular semi-conducting phase, but production of polymeric nitrogen has failed until now.
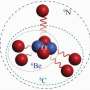
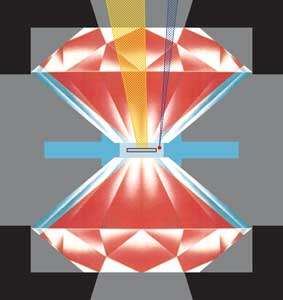
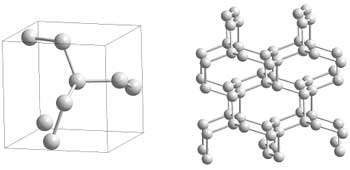
Researchers of the High Pressure Group at the Max Planck Institute for Chemistry in Mainz have now been successful: they synthesised polymeric nitrogen directly from its molecular form at temperatures above 2000 K and pressures above 110 GPa (1.1x106 atm) using a novel arrangement of the laser-heated diamond cell (Fig. 1). X-ray diffraction measurements and Raman spectra of a transparent crystal confirm the formation of polymeric nitrogen with the theoretically predicted cubic gauche structure (cg-N); see Fig. 2. The phase is a stiff substance with a bulk modulus above 300 GPa, characteristic of strong covalent solids. "Therefore, we call it nitrogen diamond", says Mikhail Eremets.
A lot more energy is stored in the single-bonded polymeric nitrogen than in the known stable form of triple-bonded molecular nitrogen. Therefore, a large amount of energy would be released under the transformation from the single-bonded to the molecular form, much more than that of the most powerful energetic materials. Since the only product of this transformation would be just common non-polluting molecular nitrogen, one should check if the new polymeric nitrogen could be used as a fuel or as an explosive. "First of all, we should try to recover the compound to ambient temperature and pressure", Eremets says.
Figures:
Fig. 1: Schematic view of a diamond anvil press. The sample in the centre is contained by a metallic gasket and an inert gas pressure medium. The sample is heated with high power IR laser (yellow) and the pressure is measured using the shift of the ruby fluorescence line which is excited with a blue argon-ion laser.
Fig. 2: Polymeric cg-N-structure: Each nitrogen atom is connected to three neighbours by three single covalent bonds.
Images: Max Planck Institute for Chemistry
Image: Max Planck Institute for Chemistry
Original publication:
Eremets, M.I., A.G. Gavriliuk, I.A. Trojan, D.A. Dzivenko and R. Boehler,
Single-bonded form of nitrogen
Nature Materials, August 2004; published online 4 July 2004,
dx.doi.org/10.1038/nmat1146
Source: Max Planck Institute for Chemistry