Scientists discover new way to look at how molecules twist and turn on water
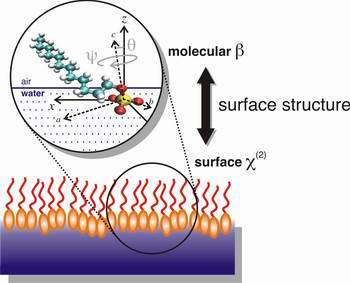
Chemists have discovered details about how the tadpole-shaped molecules found in many soaps and detergents bury their heads into the top-most surface of water, an insight expected to yield benefits such as better methods for cleaning up environmental hazards. The findings of a team led by University of Oregon chemist Geri Richmond are featured on the cover of the Sept. 8 issue of the Journal of Physical Chemistry B.
Image: Illustration by Dennis Hore, Richmond Lab, University of Oregon
"We have developed a method to determine the tilt and twist angles of these molecules at the surface, a characterization that is important for understanding how they might function in various practical applications," Richmond said. "This is a general approach that has broad implications for a variety of chemically and biologically important applications."
"With the head groups of these molecules happy to be surrounded by water molecules at the water surface and their tails preferring to stick up out of the water, extending into the air or an adjacent oily layer in the case of an oil slick," Richmond explained, "such molecules known as surfactants are some of the most pervasive and useful chemicals in the world, found in products ranging from motor oil to cosmetics. They are also key ingredients for environmental clean-up and oil recovery."
The work by Richmond, Dennis Hore, Daniel Beaman and Daniel Parks provides a picture of how these surfactant molecules orient at an aqueous surface. Theirs are the first studies to determine the detailed orientation of simple soap head groups at the water surface, using a unique combination of laser-based experiments and computer modeling. These studies add important new insights into ongoing studies in the Richmond laboratory that seek to understand how these surfactant head groups change the properties of water at aqueous surfaces.
Source: University of Oregon















