How two liquids mix at the surface: an atomic view
Whenever cream is poured into coffee, these two liquids form a homogeneous mixture, which is difficult to separate again. Other liquids, such as water and oil, do not mix, instead forming emulsions, such as salad dressing.
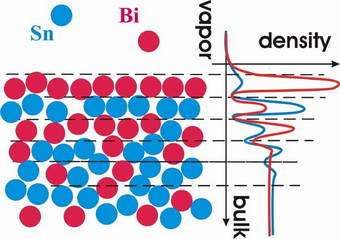
Image: Schematic representation of atomic-scale demixing observed in BiSn liquid alloy.
In results reported in this week's issue of Physical Review Letters [Phys. Rev. Lett. 95, 106103 (2005)], a collaboration lead by physicists from Harvard University have used x-rays to look at how atoms of two elemental liquids - bismuth and tin - mix together. Despite forming a perfectly miscible alloy in the bulk phase, near the surface the two elements separate into alternating atomic layers.
"The surface demixing is somewhat of a paradox since it occurs due to the strongly enhanced attraction between the atoms of the two components, while for partially miscible mixtures the opposite is true: atoms or molecules are more attracted to their own kind" explains Dr. Oleg Shpyrko, the leading author of the study.
"Surface demixing was predicted in 1950 by Defay and Prigogine, but it eluded experimentalists for more than 50 years: liquids only demix within a nanometer-deep surface region, and there are very few techniques that can probe structure of liquid surfaces on such tiny length scales. As we attempt to understand properties of nanoscale materials where most atoms are near the surface, these and other interfacial effects are expected to play a dominant role."
by Oleg Shpyrko, Argonne National Laboratory
Web address: http://liquids.deas.harvard.edu/oleg/
Email: oshpyrko_AT_anl.gov
Tel: 630-252-7540















