'Fishy' clue helps establish how proteins evolve
(PhysOrg.com) -- Three billion years ago, a "new" amino acid was added to the alphabet of 20 that commonly make up proteins in organisms today. Now researchers at Yale and the University of Tokyo have demonstrated how this rare amino acid — and, by example, other amino acids — made its way into the menu for protein synthesis. The study appeared in the December 31 advance online publication of the journal Nature.
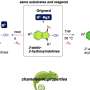
The rare amino acid the Yale researchers studied, pyrrolysine (Pyl), gave the researchers a molecular handle by being an extreme example of an amino acid that evolved to serve a highly specific need.
The amino acid alphabet shapes the language of proteins. When the genetic code was deciphered four decades ago, scientists believed that there were no more than 20 amino acid "letters" that universally meshed with the nucleic acid part of the protein code. But, like many alphabets, the language of proteins has letters with modifications — like accent marks — that modify their use.
When cells make proteins, a tightly coordinated pair of molecules — a tRNA and a tRNA synthetase — ensure that the correct amino acid is added in a growing protein chain. These molecules are highly specific for the amino acid they "manage" and are coded directly in the genome. All of the 20 common amino acids are incorporated into proteins in this way. However, only two uncommon amino acids, including Pyl, have been discovered that follow this pattern.
In most cases, an uncommon amino acid in proteins — like letters with accent marks — results from modification of one of the standard 20 amino acids after it has become part of the protein. Many human proteins are modified in this way, and deficiencies in these modifications are linked to myriad human diseases including cancer, neurodegeneration, and metabolic disorders.
"Pyl turns out to be special because it represents an uncommon amino acid that is incorporated during normal protein synthesis," said Yale postdoctoral fellow and lead co-author Patrick O'Donoghue. "This is the key difference that makes Pyl so interesting and valuable to molecular biologists. It opens the door to engineering the genetic code."
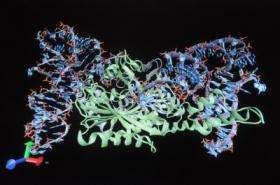
Pyl is so rare that it has been found in only seven organisms. Each of these microbes evolved in an unusual environmental niche and all use methylamines — the compounds that make fish smell "fishy" — as a source of energy. Söll's research team characterized and crystallized the molecules that "manage" Pyl and created images that show how these molecules have evolved to work together.
"This is the handle we needed to effectively produce an 'expanded' genetic code," said O'Donoghue. "Now we have the ability to directly genetically encode other uncommon amino acids. By doing that, we will be able to isolate the role of particular modifications and to begin to understand their functions and their role in human disease."
"We have found why it is probably not accidental that out of more than 300 amino acids found in natural proteins, only two have been added beyond the standard 20-member amino acid alphabet," said principal investigator Dieter Söll, Sterling Professor of Molecular Biophysics & Biochemistry and professor of chemistry at Yale.
"This work provides a tantalizing glimpse into how proteins have evolved in living cells," said Laurie Tompkins, who oversees protein synthesis grants at the National Institutes of Health's National Institute of General Medical Sciences, which partially supported the work. "The unique way in which the synthetase binds its tRNA substrate is a testament to the ancient roots of this remarkable enzyme."
Reference: Citation: Nature (31 Dec 2008), doi: 10.1038/nature07611. www.nature.com/nature/journal/ … abs/nature07611.html
Source: Yale University