Berkeley chemists pioneer low-cost water testing devices
(PhysOrg.com) -- Because of both population growth and the impact of climate change, safe drinking water will become one of the planet's most precious commodities. But public health workers lack simple, low-cost testing devices for water contaminants, especially in developing countries.
Thanks to Berkeley chemistry professor Matt Francis, that may change soon. Francis and colleagues have developed a hydrogel material that can simultaneously detect contaminants in water and remove them. The hydrogel shrinks as it absorbs heavy metal pollutants, signaling the presence of cadmium and other toxic ions, even as it absorbs them from the contaminated water.
Better yet, the hydrogel specifically targets and removes these toxic ions even from sources such as brackish water that are loaded with sodium, potassium and magnesium ions. After contamination, the hydrogel can be flushed with inexpensive chelating (metal binding) agents and reused.
The hydrogel material is described in a recent paper in the Journal of the American Chemical Society, entitled "Metallothionein cross-linked hydrogels for the selective removal of heavy metals from water." The other co-authors are Aaron Esser-Kahn, a Berkeley chemistry graduate student, and Anthony Iavarone of Berkeley's QB3/Chemistry Mass Spectrometry Facility.
"Our paper highlights the advantages of combining the function of proteins with the bulk properties of synthetic materials," says Francis. "Our system is capable of selectively sensing and removing heavy metal contaminants, and it can be recycled using solutions of inexpensive chemical chelators. It can easily be integrated into low-cost, practical tools for contaminant sensing."
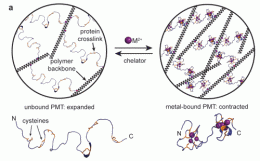
The hydrogel is based on a series of proteins that have evolved in several biological kingdoms to sequester heavy metal contaminants. Called metallothioneins, these proteins bind to the copper, zinc, cadmium, mercury, chromium, and arsenic ions that can poison water supplies. Metallothioneins act as an organism's self-defense against toxic ions such as cadmium, and may also act to regulate the levels of potentially useful ions such as copper and zinc. When these proteins bind to heavy metals, they fold and shrink.
"The material was found to bind cadmium ions in the largest amount," says Francis, "followed by copper and mercury ions. In the case of cadmium, the hydrogel was found to bind up to 4.5 percent of its dry weight in metal ions."
The hydrogel material designed by the authors consists of polymer coils held together by strands of metallothioneins. Imagine a mass of long polymer coils loosely held together by rubber bands. If the rubber bands suddenly tightened, the volume would shrink dramatically. Visually, the hydrogel shrinks in much the same way as a soft contact lens that has been accidentally left out overnight.
"What you are seeing," says Esser-Kahn, "is the effect of millions of protein strands folding and pulling the polymer chains closer together."
Like all proteins, metallothioneins are composed of a sequence of amino acids. Metallothioneins have a high frequency of the amino acid cysteine in their primary sequence, and this presents a significant challenge for incorporating them into hydrogels and other materials.
Many conventional chemical techniques for attaching proteins accomplish this by reacting with the cysteines in the protein's backbone, which prevents them from binding metals and freely changing shape. The Francis group has developed a technique to attach chemical handles to both ends of each protein chain, and to link them to hydrogels and other materials only at these positions. This leaves the proteins free to fold and change their shape in response to heavy metals or other chemicals.
For now, the authors foresee the hydrogels being used primarily for testing, not for large-scale municipal water purification. The metallothionien they chose was derived from pea plants and was expressed in E. coli bacteria in small quantities. With large fermentation facilities, the production process could be scaled to yield much larger quantities at relatively low cost.
"It's important to remember," says Esser-Kahn, "that this hydrogel was synthesized using chemistry that is independent of any particular protein sequence, so it can be used to make many different hybrid protein-polymer materials to test for several chemical compounds. We are working on incorporating proteins known to bind PCBs, dioxins, estradiol, and other persistent organic pollutants by using the same technique."
"You could imagine an array of tubes filled with thin cylinders of hydrogel, each designed to test for a different pollutant," says Francis. "A researcher in the field could add water and note the amount of contamination by measuring how much each hydrogel contracted. No electricity or complex lab equipment would be required."
In addition to his position as an Associate Professor of Chemistry at Berkeley, Francis is a faculty researcher in the Materials Sciences Division of the Lawrence Berkeley National Laboratory. This work was supported by the Director, Office of Science, Materials Sciences and Engineering Division, U.S. Department of Energy
Provided by UC Berkeley