First 3D images obtained of core component of molecular machinery used for cell reproduction
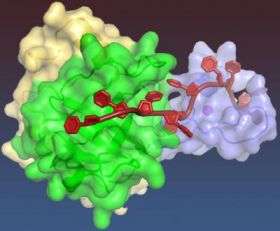
For the first time, structural biologists have managed to obtain the detailed three-dimensional structure of one of the proteins that form the core of the complex molecular machine, called the replisome, that plant and animal cells assemble to copy their DNA as the first step in cell reproduction.
The molecular structure of the protein, Mcm10, was published online by the journal Structure on Dec. 9. Its discovery was a collaborative effort by Brandt Eichman, assistant professor of biological sciences at Vanderbilt University, and Walter Chazin, Chancellor's Professor of Biochemistry and Physics at Vanderbilt, working with Anja Katrin-Bielinsky at the University of Minnesota.
Currently, the process of DNA replication in eukaryote cells – cells that have their genetic information contained in a nucleus – is a "black box." Biologists know what goes in and what comes out but they know very little about how the process actual works at the molecular level. Because form causes function in the protein world, determining the 3D structure of the 30-40 proteins that combine to form the replisome is a necessary first step to figuring out the details of this critical process and understanding how it can go wrong.
The structure of Mcm10 was determined using cells from the African clawed frog (Xenopus laevis); the structures of analogous proteins in human and other animal cells should be nearly identical, the researchers maintain. The Mcm10 structure reveals a special feature, called the OB-fold, that proteins use to interact with single-stranded DNA and a series of three loops that the researchers believe are used to clamp down on the DNA. The protein also contains a protrusion – called a zinc finger because it is built around a zinc atom – that proteins normally use to recognize specific double-stranded DNA segments. In this case the zinc finger appears to be modified in a way that allows it to detect generic DNA.
The researchers think that Mcm10 may play a role in positioning the other proteins in the replisome onto the single DNA strand so that it may be correctly read and duplicated, while acknowledging that they have very little information about how it functions.
Source: Vanderbilt University















