Scientists drill holes through deadly bacteria's Kevlar-like hide
(PhysOrg.com) -- To protect themselves from human defenses, disease-causing bacteria have evolved a cell wall made from a nearly impenetrable tangle of tightly woven strands. That’s made it difficult for scientists to see what goes on inside these potentially deadly organisms. But that era is now over.
In research published in the Proceedings of the National Academy of Sciences, Rockefeller University researchers have figured out how to drill holes through the Kevlar-like hide of gram-positive bacteria without obliterating them, and in doing so, they’ve made it possible to study, from the inside out, most of the known bacteria on the planet.
The work, led by Vincent A. Fischetti, head of the Laboratory of Bacterial Pathogenesis and Immunology, provides, for the first time ever, a look inside the rapidly multiplying and highly contagious Streptococcus pyogenes, the culprit behind a myriad of diseases, including strep throat and rheumatic fever. At a time when organisms are increasingly acquiring “superbug” powers, Fischetti and his colleague Assaf Raz, a graduate student in the lab, have used the technique to look specifically at a well-known enzyme called sortase A and its distribution inside the cell. Common to all gram-positive bacteria, the enzyme functions by anchoring surface proteins to the cell wall, endowing the bacteria with their infectious properties.
“If you interfere with this process, you get naked bacteria and naked bacteria are unable to cause infection,” says Fischetti. “So the idea here is that the more we know how sortase functions inside the cell, the more strategies we’ll have to interfere with its activity stripping the bacteria of their pathogenic surface proteins.” Although the researchers worked with S. pyogenes, the approach could work on any gram-positive bacteria such as methicillin-resistant Staphylococcus aureus, or MRSA, which is increasingly becoming resistant to even our strongest antibiotics.
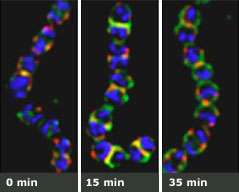
The technique relies on enzymes produced by viruses, called bacteriophages, which attack only bacteria. Unlike antibiotics, which take time to take effect, phage enzymes strike with blitzkrieg speed, preventing bacteria from mustering a defense. Usually, these enzymes destroy their target, leaving nothing but cellular debris behind. That’s because the pressure inside a bacterium is like a champagne bottle: Once it’s opened, it explodes. In their work, however, Fischetti and Raz figured out how to poke holes in S. pyogenes while keeping the bacteria intact. These holes provide an entryway for tags that fluoresce when they attach to molecules inside the altered bacteria, allowing scientists to visualize, from the inside out, what makes these single-celled powerhouses infectious.
In the past, if scientists wanted to study what goes on inside bacteria, they were largely limited to working with nonpathogenic types whose cell walls could be punctured with established methods. The new technique, however, allows them to directly study pathogenic bacteria and ask specific questions about them.
Fischetti and Raz were interested in whether the distribution of sortase A inside the cell affects the distribution of protein M, one of many surface proteins found on these bacteria. The researchers found that as the bacteria divide, the tagged sortase A assembles at a very specific location: the point of cell division where it anchors protein M. Interestingly, before the bacterium finishes dividing, sortase A starts to assemble at the new point of division — even before the recently formed bacteria starts dividing.
“So early assembly of sortase A at the division site allows bacteria to attach surface proteins to the cell wall as it is being built,” says Raz. “Whether or not sortase A is related to the division machinery we do not know yet, but we now have the tools to try and find out.”
Perhaps this migration is a way for bacteria to be ultra-organized. “Strep divide every 20 or 30 minutes under optimal conditions,” says Fischetti. “During that time, a lot of things are going on and the bug has to be extremely organized for all these things to happen very quickly. We now have the tools to start answering how these organisms carry out this feat. That’s one important thing that this work has accomplished. It could help us understand what makes this and other disease organisms function.”
Citation: Proceedings of the National Academy of Sciences 105 (47): 18549–18554 (November 25, 2008)
Provided by Rockefeller University



















