Hope for Alzheimer’s patients? Dipeptide blocks the formation of toxic amyloid β-peptide aggregates in mice
(PhysOrg.com) -- Alzheimer's disease is the primary cause of age-related dementia. About 15 million people are affected by this neurodegenerative disease worldwide. It has so far not been possible to combat the causes of Alzheimer’s disease. Israeli researchers have now developed a novel approach for treatment. As they report in the journal Angewandte Chemie, the new drug, a molecule made from two nonphysiological amino acids, improves the cognitive abilities of mice with Alzheimer’s and reduces the amyloid plaques in their brains.
Many years before clinical symptoms appear, threadlike deposits of incorrectly folded amyloid β-peptides, known as plaques, form in the brains of Alzheimer’s patients. It was previously assumed that these plaques initiate the degeneration of nerve cells; however, more recent discoveries indicate that smaller, soluble aggregates of the amyloid-forming peptide are the actual cause for the loss of learning and memory functions.
These oligomers of about twelve peptide units have a strong toxic effect on nerve cells. The new therapeutic approach taken by researchers working with Ehud Gazit at the University of Tel Aviv aims to block the formation of these toxic oligomers.
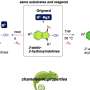
A drug molecule was rationally designed that contains an aromatic amino acid and a β-sheet breaker. Aromatic side-groups play a key role in the aggregation of amyloid-forming peptides; the drug should thus bind to the aromatic core of the β-amyloid peptide through its aromatic element. Also, in amyloid aggregates, the proteins adopt the form of β-sheets that are folded like accordions; the drug should thus be a β-breaker, a molecule that prevents proteins from folding into β-sheets. In addition, the molecule must be small so that it can be absorbed by the digestive tract after oral ingestion. It must also not be rapidly degraded by the body and must be nontoxic.
The small dipeptide—a molecule made of two amino acids—developed by Gazit’s team meets all of these requirements: the nonphysiological amino acid α-aminoisobutyric acid acts as a β-breaker, and the second amino acid, D-tryptophan, contains an indole group, which is an effective binder of aromatics. At the same time, D-tryptophan stabilizes the dipeptide, because D-amino acids are broken down more slowly in the body than are the physiological L-amino acids.
When genetically altered mice with Alzheimer’s disease are treated with the dipeptide, their disrupted cognitive functions normalize. A significant decrease in the concentration of amyloid-forming β-peptides and in the size of the plaques was found in the brains of these mice.
Citation: Ehud Gazit, Cognitive-Performance Recovery of Alzheimer's Disease Model Mice by Modulation of Early Soluble Amyloidal Assemblies, Angewandte Chemie International Edition, doi: 10.1002/anie.200802123
Provided by Wiley