Researchers turn one form of adult mouse cell directly into another
(PhysOrg.com) -- In a feat of biological prestidigitation likely to turn the field of regenerative medicine on its head, Harvard Stem Cell Institute (HSCI) co-director Doug Melton and post doctoral fellow Qiao "Joe" Zhou report having achieved what has long been a dream and ultimate goal of developmental biologists – directly turning one type of fully formed adult cell into another type of adult cell.
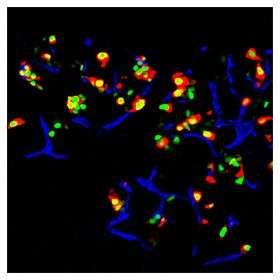
The Melton team reports in today's online edition of the journal Nature that, using a technique it is calling "direct reprogramming," the team is able to turn mouse exocrine cells, which make up about 95 percent of the pancreas, into precious and rare insulin-producing beta cells. These beta cells, which comrpise about one percent of the pancreas, are the cells that die off in Type I diabetes.
In addition to its value for the field of regenerative medicine, the work also is a major step forward toward eventually developing a treatment for Type II – and eventually Type I – diabetes, a treatment that might someday eliminate the need for patients to constantly monitor their blood sugar and take insulin-adjusting medications, or even insulin. It is important to note, however, that there are numerous scientific hurdles that lay ahead before a treatment could be tested in humans.
Melton, a Howard Hughes Medical Institute Investigator, has discussed the work in general terms at a few scientific meeting over the course of the last few months, and his talks have generated expressions of surprise from those who have heard them, or even heard about them.
George Q. Daley, immediate past president of the International Society for Stem Cell Research and a member of HSCI's Executive Committee, said Melton's findings are of a caliber that "will revolutionize what is already a revolutionary field."
Unlike the process involved in creating induced pluripotent stem cells (iPS), which have caused enormous excitement ever since their introduction two years ago by Japanese researcher Shinya Yamanaka, this direct reprogramming technique does not require turning adult cells into stem cells and then figuring out how to induce them to differentiate into a desired cell type. Melton emphasized, however, that direct reprogramming does not in any way eliminate the need for, or value of, work with iPS cells or human embryonic stem cells. "We need to attack problems from multiple angles," said Melton, stressing that his lab is using several approaches and will continue to work with iPS and hES cells.
Sir John Gurden, the internationally renowned developmental biologist under whom Melton did his graduate work at Oxford University and the first scientist to successfully clone an adult mammal - a frog, said that "What you really want is a missing cell type, one that is not functioning properly to be derived from something else. But you only want that cell type. So I think this is a really important step forward in exercising what people really wanted and showing how well it can work, by this gene over-expression procedure."
As is the case with all iPS work thus far, Melton's experiments involved using viruses to integrate the transcription factors into the target cells. Because of the risks that approach would pose to humans, the team is looking for chemicals that might effectively and, most important, safely replace the viruses.
Joan Brugge, Chair of the Department of Cell Biology at Harvard Medical School, said the new study "provides exciting new insights into yet another aspect of cell plasticity that was not appreciated previously and that offers great potential therapeutically. Direct reprogramming represents a more straight-forward strategy to treat diseases involving loss of function of specific cell populations than approaches requiring an intermediate embryonic stem cell," she said.
Whitehead Institute stem cell researcher Rudolf Jaenisch, who has heard one of Melton's presentations on the new work, said that there had previously been so much empty talk about supposedly successful direct reprogramming efforts that if anyone other than Doug Melton were publishing this report Jaenisch would view it quite skeptically.
In fact, even Melton was "more than a little surprised to find that we could use a combination of just three transcription factors to reprogram one cell type into another." He said that first author Joe Zhou's experiments "combined a systematic approach to identifying the relevant transcription factors, hard work, and a bit of luck."
Luck, serendipity, is virtually always a part of the process of scientific discovery. But the choice of transcription factors Ngn3, Pdx1, and MafA, while lucky, could hardly be said to have resulted from luck. Instead, it was the product of two years of repetitive lab work that began, Melton said, "by asking what genes you have to have turned on in the cell for it to become a beta cell.
"If you want to do reprogramming it doesn't take great insight to figure out that the key genes are transcription factors – the proteins that bind DNA and tell cells which genes to turn on and which to turn off," said Melton, the co-chairman of Harvard's new Department of Stem Cell and Regenerative Biology.
Melton likened the the multi-step process a stem cell goes through during differentiation into a specific adult cell type to passing through a series of doors. "There are locks on all the doors," he said, "and the locks are transcription factors. We asked which ones are present in the beta cell, and that gave us 1,100 transcription factors to choose from. Eventually we learned that of the 1,100, only about 200 are actually expressed in cells that are involved in forming the pancreas.
"Next," Melton continued, "we decided that of the 200, we only cared about the ones that are expressed in the key part of the pancreas where the beta cells are – and that got us down to about 28. Then we did some lineage studies," he explained, "and we got it down to nine. Joe said, 'my best guess is it's these nine.' And he were right. It was a messy experiment, mixing all nine and injecting them into the pancreas. Then we found out that it got better and better as we removed one gene at a time from the nine, and eventually we found that it actually works best with three transcription factors – that six of them aren't that important. And that's the fun of science!" Melton said, a grin spreading across his face.
But back to serendipity for a moment:
Suppose the experiment hadn't worked with those nine transcription factors; what then? "If it hadn't worked with those nine, we'd probably have dropped the experiment and gone onto something else; there would have been just too many possible combinations of transcription factors to wade through," Melton said.
"We’re intrigued by the possibility that this approach, which has worked for pancreatic insulin-producing cells, could be more widely applied to many kind of cells, especially those that are lost in disease or following injury," Melton said. "And at the same time, we are exploring the possibility of using this general approach in a clinical context to make new beta cells for patients."
Audio: www.news.harvard.edu/multimedi … io/080826_melton.mp3
Provided by Harvard University