Catalyst for water oxidation adopted from plants: a means for energy-efficient production of hydrogen?
(PhysOrg.com) -- Hydrogen will be one of the most important fuels of the future. It would be ideal to obtain hydrogen by splitting water instead of from petroleum. However, the electrolysis of water is a very energy intensive process, making it both expensive and unsustainable if the electricity necessary to generate it comes from the burning of fossil fuels. Photolysis, the splitting of water by light, is a highly promising alternative.
A team of Australian and American researchers has now developed a catalyst that effectively catalyzes one of the necessary half reactions, the photooxidation of water. As it reports in the journal Angewandte Chemie, the core of the catalyst is a manganese-containing complex modeled after those found in photosynthetic organisms.
Electrolysis is the reverse of the process that occurs in a battery: that is electrical energy is converted to chemical energy. The electrolysis of water involves two half reactions: at the cathode, protons (positively charged hydrogen ions) are reduced to hydrogen, whereas at the anode the oxidation of water produces oxygen.
The goal of the researchers is to use sunlight to get this energy-intensive process going. To make this work, the light-harvesting power of modern solar cells must be combined with effective photocatalysts for the oxidation of water and reduction of hydrogen ions into hydrogen gas.
The biggest hurdle to overcome in the photocatalytic splitting of water to date has been the lack of a robust catalyst that oxidizes water. In fact, the best known catalyst, which very effectively oxidizes water when irradiated with visible light, is a manganese-containing enzyme in the photosynthetic apparatus of living organisms.
Robin Brimblecombe and Leone Spiccia at Monash University (Australia), Gerhard F. Swiegers at the Commonwealth Scientific and Industrial Research Organisation (CSIRO, Australia), and G. Charles Dismukes at Princeton University (USA) have used this structure as a model for their photocatalyst.
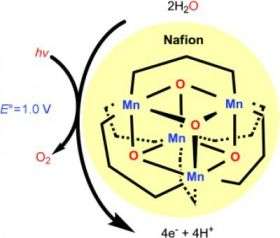
The catalyst in question is a manganese oxo complex with a cubic core made of four manganese and four oxygen atoms capped by ancillary phosphinate molecules. The catalytically active species is formed when energy from light causes the release of one the capping molecules from the cube.
However, the manganese complex is not soluble in water. The researchers have overcome this problem by coating one electrode with a wafer-thin Nafion membrane. Housed within the aqueous channels of this membrane, the catalytic species is stabilized and has good access to the water molecules. Irradiation with visible light under an applied 1.2 volts leads to the effective electro-oxidation of water.
This anodic half-cell could be easily paired with a catalytic hydrogen-producing cathode cell. This would result in a photoelectrochemical cell that produces pure hydrogen and oxygen from water and sunlight.
Citation: Leone Spiccia, Sustained Water Oxidation Photocatalysis by a Bioinspired Manganese Cluster, Angewandte Chemie International Edition 2008, 47, No. 38, doi: 10.1002/anie.200801132
Provided by Wiley
















