'Yanking' Chemical Bonds with Molecular Wires Speeds Reactions
Using a chain of molecules as an infinitesimal lanyard to tug on a chemical bond about to break, Duke University chemists have found they can speed a complex chemical reaction.
Their unusual manipulative technique can reveal previously unknown details about the evolution of such two-step bond reactions, said assistant Duke chemistry professor Stephen Craig. It might ultimately aid efforts to develop new kinds of polymers that can "heal" themselves after tearing, he said.
Craig, current doctoral student Farrell Kersey and former graduate student Wayne Yount described their discoveries in a research paper published online Friday, March 3, 2006, in the Journal of the American Chemical Society (JACS). The work was funded by the National Science Foundation.
"We probed a reaction in which a bond was being made and a bond was being broken by pulling on the bond being broken with an atomic force microscope (AFM)," said Craig. An AFM detects forces or creates images of surfaces at molecular scales by mechanically probing with a flexible microscopic cantilevered tip.
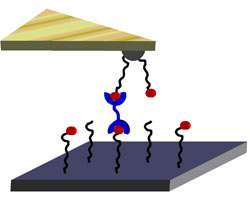
In their experiments, Craig's group used an AFM tip to exert almost infinitesimally small tugs on a molecular complex made of pyridine and the metal palladium.
The researchers dangled the pyridine-palladium complex in space as if it were part of a molecular trapeze act, by attaching trapeze "wires" made of atomic chains of the molecule polyethylene glycol (PEG). One PEG chain connected the dangling pyridine-palladium to the AFM's tip. A separate PEG "wire" anchored the complex underneath onto an underlying surface substrate.
When the AFM's flexible tip pivoted upward, it pulled on the bond linking the pyridine to the palladium. "This is almost like spring-loading that bond," Craig said.
"As a bond breaks, it stretches," he said. "The distance between the atoms gets further and further. And we could infer from the behavior of this experiment that the rate of the reaction speeded up."
Since the whole array was submerged in a solution of the chemical solvent DMSO, the bond was already under pressure before the AFM began its work, he said.
"Because this solvent was present in excessive amounts, it wanted to form a bond with the palladium," he said. But the nature of that reaction requires the DMSO-palladium bond to form first before the palladium and pyridine could sever their connection, he added.
The Duke chemists sought to study how the sequence of bond forming and breaking would be affected if they artificially stretched the palladium-pyridine bond towards the breaking point.
They found that, although the pace of the reaction was accelerated, the order of bond forming and breaking did not change. "We could spring-load the bond enough so it sought to break very quickly. But the reaction still waited for the DMSO to bond to the palladium before the pyridine came off," he said.
The researchers also found that, when they repeated the experiment with a palladium-pyridine complex incorporating a modified pyridine, the response to pulling on the bond was the same even though the energy levels needed for bond-breaking were different.
These findings "are absolutely consistent with some very fundamental notions about the way energy is exchanged in chemical reactions," Craig said. "But to my knowledge it's not an experiment that anyone else has done to test whether that was the case. This could lead to a more sophisticated understanding of the way reactions happen at their most fundamental levels."
According to Craig, additional studies into the order and consequences of chemical bond-breaking might also aid the discovery of new materials. "Someone might try to design certain types of molecules that would respond to mechanical stresses by breaking in a way that's desirable," he said.
For example, he said such research might aid researchers like him who work on "self-healing polymers." Those are molecules in the early stages of development that would release chemicals to repair newly formed tears and cracks.
Source: Duke University















