Clean, carbon-neutral hydrogen on the horizon
Hydrogen as an everyday, environmentally friendly fuel source may be closer than we think, according to Penn State researchers.
"The energy focus is currently on ethanol as a fuel, but economical ethanol from cellulose is 10 years down the road," says Bruce E. Logan, the Kappe professor of environmental engineering. "First you need to break cellulose down to sugars and then bacteria can convert them to ethanol."
Logan and Shaoan Cheng, research associate, suggest a method based on microbial fuel cells to convert cellulose and other biodegradable organic materials directly into hydrogen in today's (Nov. 12) issue of the Proceedings of the National Academy of Sciences online.
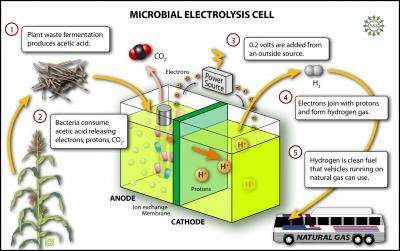
The researchers used naturally occurring bacteria in a microbial electrolysis cell with acetic acid – the acid found in vinegar. Acetic acid is also the predominant acid produced by fermentation of glucose or cellulose. The anode was granulated graphite, the cathode was carbon with a platinum catalyst, and they used an off-the-shelf anion exchange membrane. The bacteria consume the acetic acid and release electrons and protons creating up to 0.3 volts. When more than 0.2 volts are added from an outside source, hydrogen gas bubbles up from the liquid.
"This process produces 288 percent more energy in hydrogen than the electrical energy that is added to the process," says Logan.
Water hydrolysis, a standard method for producing hydrogen, is only 50 to 70 percent efficient. Even if the microbial electrolysis cell process is set up to bleed off some of the hydrogen to produce the added energy boost needed to sustain hydrogen production, the process still creates 144 percent more available energy than the electrical energy used to produce it.
For those who think that a hydrogen economy is far in the future, Logan suggests that hydrogen produced from cellulose and other renewable organic materials could be blended with natural gas for use in natural gas vehicles.
"We drive a lot of vehicles on natural gas already. Natural gas is essentially methane," says Logan. "Methane burns fairly cleanly, but if we add hydrogen, it burns even more cleanly and works fine in existing natural gas combustion vehicles."
The range of efficiencies of hydrogen production based on electrical energy and energy in a variety of organic substances is between 63 and 82 percent. Both lactic acid and acetic acid achieve 82 percent, while unpretreated cellulose is 63 percent efficient. Glucose is 64 percent efficient.
Another potential use for microbial-electrolysis-cell produced hydrogen is in fertilizer manufacture. Currently fertilizer is produced in large factories and trucked to farms. With microbial electrolysis cells, very large farms or farm cooperatives could produce hydrogen from wood chips and then through a common process, use the nitrogen in the air to produce ammonia or nitric acid. Both of these are used directly as fertilizer or the ammonia could be used to make ammonium nitrate, sulfate or phosphate.
Source: Penn State



















