The nanoworld of corrosion
The effect of corrosion has an impact on about 3% of the world's Gross Domestic Product. From a positive point of view, however, chemical attack of metal surfaces may result into surface nano-structures with very interesting technological applications such as catalysts and sensors. Therefore, a better understanding of corrosion processes is required to both prevent it and make the most of it.
Scientists from Germany and the European Synchrotron Radiation Facility (ESRF) have highlighted a self-organization process on the surface of a metal alloy, which is of crucial importance in determining the response to corrosion of this material. In fact, this study, providing a structural description with atomic-scale resolution thanks to the X-rays from the ESRF synchrotron, unvealed the chemical composition and structure of a protective surface layer which hinders further corrosion. The authors publish their results in Nature this week.
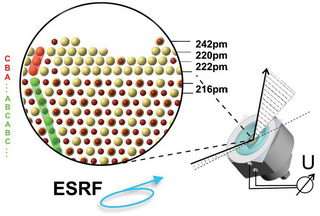
Researchers from Max Planck Institute, the University of Ulm (Germany), and the ESRF used the European synchrotron light source to reproduce in situ the onset of the corrosive process in a gold-copper alloy. Gold is a very noble metal, which doesn't corrode, whilst copper is less noble and, thus, more prone to chemical attack. At the first moments of corrosion, the copper-gold alloy develops a mechanism to protect itself with an extremely thin gold-rich layer.
This layer has an unexpected crystalline and well-ordered structure. When the corrosion process proceeds, this alloy layer transforms into gold nano-islands of 20 to 1.5 nanometres. These islands eventually develop into a porous gold metal layer, which may have technological applications: "Understanding and controlling the formation of the first layer and the nano-islands may help to produce nano-materials with specific properties", explains Jorg Zegenhagen, one of the authors of the paper.
In order to carry out these experiments, researchers placed the samples in an electrochemical cell filled with sulphuric acid, in which voltage can be applied, and monitored the early corrosion process. "We found a vast amount of detail on structural evolution and chemical information by combining detailed 3D analysis of the structure with additional anomalous scattering experiments before more severe corrosion happened", explains Frank Renner, first author of the paper.
These new insights can be applied to a variety of different alloys used in corrosive environments and to materials that can exploit such degradation to form porous metals of technological interest. Although understanding the process of corrosion in gold-copper alloy has only become possible now, the process itself is many centuries old. Ancient Incas metal-smiths stretched their supplies of precious gold by mixing it with copper, and then surrounding the alloy with salty substances. This created an acidic environment that dissolved the copper from the top layer, leaving a gold-rich surface ready for polishing.
Source: European Synchrotron Radiation Facility


















