Two super heavy elements discovered
A Swiss research group has participated in the discovery of new chemical elements. The elements have the numbers 113 and 115 and were discovered by a combination of physical and chemical techniques in the Russian nuclear research centre in Dubna. With its radiochemical expertise the Paul Scherrer Institute was central to the experiment’s success.
Chemistry is currently pushing the boundaries of the scientific unknown. Until 1940 Uranium was the heaviest known element. This naturally occurring metal has the periodic number 92, as it has 92 positively charged protons in its nucleus. Since then over twenty elements with higher atomic numbers have been discovered.
The birth of element 115
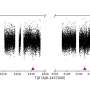
Heavy elements decay by emitting charged helium atoms - alpha particles. Such decay chains were used by American, Russian and Swiss scientists to physically prove the existence of elements 115 and its decay product after emission of the first alpha particle – element 113. In order to synthesize the atoms of element 115 a rotating target disc of americium was bombarded with a calcium beam. In a fusion reaction between target and beam particles element 115 was born. However their formation was not sufficient to prove the element’s existence as its atoms only lived a mere tenth of a second and were difficult to detect. The radiochemical experiment proved much more successful as it yielded five times as many atoms.
Radiochemical proof
As expected, the element 115 decayed by emitting an alpha particle to become element 113 and then in further emissions of four alpha particles, to dubnium, element 105. It was here that the elegant experimental approach from the PSI came into play. Behind the rotating americium disc (target) a copper plate was placed which collected all element 115 atoms emitted from the target. The copper plate was chemically processed by means of liquid chromatography techniques and 15 atoms of dubnium (which have a half-life of 32 hours) were observed. The decay pattern of these atoms supported the evidence of the physics experiment. Thus the discovery of element 115 and its progeny, element 113, was proven. All elements below atomic number 113 are already known.
“Switzerland can celebrate a scientific first, even when the experiment was performed abroad”, commented Heinz Gäggeler, leader of the Swiss research group, Head of the Department of Particles and Matter at the PSI and Chemistry Professor at the University of Berne. It is the first time Switzerland has been at the forefront of the race to expand the periodic table.
Source: Paul Scherrer Institut