Membrane fusion at the synapse: Janus faced synaptotagmin-1 helps to keep the fast pace
Imagine a bathtub with two soap bubbles colliding but never fusing. Then you add detergent, and the surface of the water goes flat as the walls of the bubbles collapse and merge.
Dr. Christian Rosenmund, professor of neuroscience and molecular and human genetics at Baylor College of Medicine, and graduate student Mingshan Xue use that analogy to describe the action of synaptotagmin-1, which acts to catalyze the fusion of the membranes of tiny neurotransmitter-filled bubbles called vesicles with the wall membrane of a neuron. This action allows signals to flow between neurons.
In a report in the current issue of Nature Structural and Molecular Biology, Rosenmund, Xue and colleagues from The University of Texas Southwestern Medical School at Dallas turn the notion of how synaptotagmin-1 accomplishes this task upside down, making an important step forward in understanding how synaptotagmin-1 accomplishes this task.
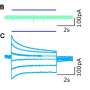
In fact, said Xue, bringing the two membranes together involves both the top and bottom of a key domain of the protein. He demonstrated this in a series of elegant experiments that validated the importance of the bottom of the domain.
"We are looking at the molecular mechanism of synaptic transmission or how neurons communicate in the brain," said Xue.
Previously, experts had thought that only the top areas of the so-called C2B domain of synaptotagmin-1 were involved in facilitating fast neurotransmitter release.
When the neuron is in a pre-synapse phase, it explodes in electrical activity that opens a channel allowing positively charged calcium ions to go to the balloon-shaped vesicles inside the presynapse to trigger the release of neurotransmitter. Transferring neurotransmitters from one neuron to another requires the fusion of the vesicle's membrane with its host plasma membrane. This allows the neurotransmitter to diffuse to the postsynaptic part of the synapse.
Neurons send and receive information via two structures that make up the synapse. On the sending site, the electrical activity of the neuron needs to be rapidly converted into a chemical signal via release of a hormone or neurotransmitter.
The trigger for this is the flux of calcium ions into the nerve terminal. The receiving neuron on the other site of the synapse detects the neurotransmitter via receptors and converts it again into electrical activity.
It takes no more than 1/1,000th of a second for this to occur. Neuroscientists are not only fascinated by this astonishing speed, but they also recognize that disturbing the speed of synaptic transmission has deleterious consequences on brain function and can lead to various diseases of the nervous system including schizophrenia, depression, Parkinson's disease, epilepsy and Alzheimer's disease.
Rosenmund and colleagues found that synaptotagmin-1 plays an important role in maintaining this speed. Bringing two fusing membranes close enough so that they fuse with each other is a crucial step in the process. The housekeeping machinery that accomplishes this in many biological processes is made up of the so-called SNARE proteins that form protein bundles across vesicle and plasma membranes, hurling the vesicle or balloon-like structure close to the membrane. While this process works very well, it is just too slow for synapses operating in a millisecond.
"That's where synaptotagmin-1 and calcium come into the game," said Rosenmund. "Its C2B domain binds at the top site to calcium ions, allowing the subsequent attachment to of the two membranes."
In their current work, Xue, Rosenmund and colleagues showed that the bottom of the C2B domain is also critical in bringing the membranes of the neuron together with the vesicle's membrane, allowing the release of neurotransmitter.
"Nature invented SNARE first to help the process," said Xue. "But that did not take into account the high demand of the neurons. When the calcium comes, we need a fast neurotransmitter release. Nature then invented synaptotagmin-1 to respond to the calcium. It's a trigger. The molecule is turned on and interacts with both membranes."
Source: Baylor College of Medicine