‘Defective’ Nanostructures Make Breaking Water to Extract Hydrogen Easier
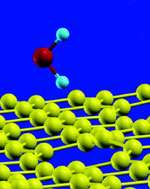
Scientists at North Carolina State University have discovered a nanoscale method for extracting hydrogen from water that requires only half the energy of current hydrogen production methods.
The researchers discovered that “defective” carbon nanotubes make it easier to “break” water molecules and extract hydrogen.
The discovery could have big implications, namely, lower hydrogen production costs, for industries looking to hydrogen as an alternative fuel.
The scientists – NC State Department of Physics professor Dr. Marco Buongiorno-Nardelli; Dr. Keith Gubbins, W.H. Clark Distinguished University Professor of Chemical and Biomolecular Engineering; post-doctoral researcher Milen Kostov; and students Erik Santiso and Aaron George – published their results in the Sept. 30 edition of Physical Review Letters.
Carbon nanotubes are structures so small that it would take 1,000 of them stacked on top of one another to equal the thickness of a human hair. The nanotubes have many potential useful applications, one of them being the ability to facilitate chemical reactions. Buongiorno-Nardelli’s team discovered that naturally occurring defects in the nanotubes can increase the rate of a chemical reaction, because the atoms that form the defective nanotubes are essentially “incomplete,” thus making them more reactive.
“Normally, when you talk about chemical reactions in carbon nanotubes, you’re imagining that these reactions are happening in perfectly formed nanostructures,” said Buongiorno-Nardelli. “But the reality is that these structures have defects – places where the carbon atom network is broken. And these defects can influence the chemical reaction.”
And that is what the scientists discovered when they began running computer models to simulate what would happen if they used the defective nanostructures to break water molecules. The current method for extracting hydrogen from water involves heating water molecules to 2,000 degrees Celsius. The high temperature “breaks” the molecule, and hydrogen is released.
“We studied water for many months and ran many different calculations, and we ended up showing that if you want to break a water molecule, you spend a lot less energy if you do it on this defective carbon material than if you do it by simply heating the molecule until it breaks,” Buongiorno-Nardelli said. “You can reduce the energy necessary by a factor of two – you can do it at less than 1,000 degrees.”
However, there are still problems to solve before a truly catalytic process can be devised – for example, how to make this dissociation reaction a viable process for hydrogen production. The team hopes to collaborate with other scientists to design and construct a nanoscale chemical reactor that will one day lead to a cost- and energy-efficient way to produce hydrogen.
“We think that nanotechnology can be used to produce more and better energy in an environmentally friendly way,” says Buongiorno-Nardelli. “Our experience with the water molecules so far leads me to believe we’re headed in the right direction.”
Source: North Carolina State University