Lost lake sheds light on past and future water security
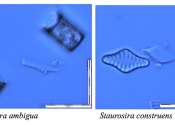
Nestled high in the Eastern Lesotho Highlands, scientists have uncovered fascinating evidence of an ancient mountain lake that flourished thousands of years ago. This discovery, made by Professor Jennifer Fitchett from the ...