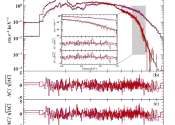
How does light interact with matter at extreme intensities, near the Schwinger limit?
The experimental generation of increasingly intense light beams could help to unveil new physical regimes occurring in the presence of very strong electromagnetic fields. While some progress has been made towards this goal, ...