Embryonic Heart Cells Thrive Only in an Environment That's Just Right
(PhysOrg.com) -- Cellular engineers at the University of Pennsylvania have determined that cardiomyocytes, the specialized cells that form the heart muscle, thrive when cultured in an environment that mimics their own elastic nature but falter, weaken or die when “grown” on stiffer or softer materials.
The study’s methods and analyses demonstrate that individual heart cells, similar in character to those derived from embryonic stem cells and induced pluripotent stem cells, are affected by physical forces at the cellular level and require the proper myocardial environment to grow and potentially repair damaged heart muscle, a key goal of stem cell and cardiovascular research. It also highlights the need for stem cell science to focus on physical parameters such as fibrosis as well as the mechanics of microenvironments to optimize cell therapy and new muscle growth.
In healthy myocardium, cardiomyocytes attach to a collagen-based extracellular matrix that must be sufficiently flexible for actomyosin forces to pump the heart. The elasticity of the extracellular matrix is an insoluble cue for many cells, influencing cell shape, protein expression and organization, as well as differentiation.
Dennis Discher and a team from Penn’s School of Engineering and Applied Science and School of Medicine isolated cardiomyocytes and placed them on substrates of varying flexibility made with hydrogels.
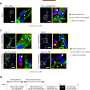
Stiffer substrates, the kind that mimic fibrotic scarring, produce heart cells that overstrain themselves and fail to stitch their proteins together to form heart muscle. The cells deform, but the actin filaments eventually pop free like shards of metal in an overworked engine. The resulting cells form unhealthy myofibrils and progressively lose their rhythmic beating.
On very soft substrates, the cells beat for days in culture but, much like a body builder with too little weight on the bench press, have done too little work to form the toned muscle and striated fibers for healthy muscle.
The perfect substrate, one that mimics the elasticity of normal heart tissue, provides an environment optimal for transmitting contractile work to the matrix and for promoting actomyosin striation and thus healthy and mature heart fibers. The matching strains between cell and matrix allow for proteins to piece together properly.
The team employed a “cysteine shotgun” as a structural marker to tag cellular proteins that experience structural changes, implying dynamic differences in intracellular protein structures that depend on the stiffness of the substrate.
The research builds on prior studies by the Discher Lab, which demonstrated that undifferentiated stem cells will differentiate depending upon the elasticity of the substrate on which they are grown. In a previous study, Discher’s team showed that stem cells placed on a stiff substrate like an infracted heart would mimic the stiffness and develop the characteristics not of heart cells, but of bone. Both studies reinforce the notion that stem cell differentiation is highly sensitive to matrix elasticity.
The study was inspired by ongoing research at Penn into the physical forces of cells and the well-known fibrotic rigidification and impairment of cardiac output that follows a heart attack or other trauma to the heart muscle.
“Embryonic heart cells are independent and self-contained, with all the proteins to contract, divide and repair themselves,” Christine Carag-Krieger, a doctoral candidate and lead co-author of the study, said. “However, prior research has determined that scarring of heart tissue, such as that occurring during a heart attack, inhibits the cell’s ability to put heart muscle myofibers back together with its usual series of protein interactions.”
The research, supported by the National Institutes of Health, the Ashton Foundation Pre-doctoral Fellowship Fund and an NIH-NHLBI Training Grant Fellowship, appeared in the Journal of Cell Science.
Provided by University of Pennsylvania