Scientists characterize protein structure of environmentally friendly bacteria
Scientists at the U.S. Department of Energy's Argonne National Laboratory have determined the structure of a key protein domain in a bacterium that could help with bioremediation of uranium-contaminated land sites.
The researchers, led by Argonne senior biophysicist Marianne Schiffer, characterized the structure of one of the principal domains in a protein responsible for certain types of movement exhibited by the bacterium Geobacter sulfurreducens.
Geobacter lives in predominantly low oxygen environments and generates energy by transferring electrons to various metallic electron-accepting atoms such as iron or uranium. This ability suggests that Geobacter might be used for remediation of certain types of hazardous waste. For example, when uranium is reduced by this process to its insoluble form, it no longer leaks into groundwater and engineers can inexpensively remove the precipitated uranium.
To get to regions of high nutrient concentration (or to escape from harmful substances), certain types of bacteria use a mechanism called chemotaxis. For chemotaxis to work reliably, the cell must be able to convert external chemical information into internal chemical processes – this process is known as signal transduction. "One of the big questions in biology is how signals get from outside the cell to inside the cell," Schiffer said.
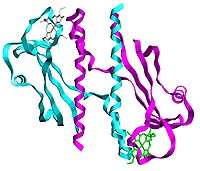
The researchers determined the three-dimensional structure of a sensory domain of a membrane-spanning protein which they believe is involved in signal transduction. Schiffer and her colleagues were particularly interested in this domain because it contains heme, a molecular component that is common in oxygen transport proteins, such as hemoglobin, or in other proteins involved in respiration or photosynthesis. While other sensor proteins that contain heme have also been described, this is the first example of a sensor protein that contains a heme covalently bound to the protein, Schiffer said.
Although Schiffer and her colleagues have not yet identified the stimulus to which this protein responds, "compiling a library of similar bacterial protein structures may at some point give researchers a better view into the molecular mechanisms that control bacterial behavior," Schiffer said. "We don't know how to determine what the domain does in the bacteria, but it is part of understanding the general picture. This protein belongs to a relatively new family of structures that allow us to gain information about what these sorts of proteins could do."
Other key researchers on the team include Argonne biologists Raj Pokkuluri and Yuri Londer and biologist Carlos Salgueiro from the Universidade Nova de Lisboa in Portugal. Part of this research was performed at Argonne's Advanced Photon Source. This work is part of a larger project on Geobacter at the University of Massachusetts-Amherst, where Derek Lovley is the principal investigator.
The results appeared in the April 11 issue of Journal of Molecular Biology and can be found online at doi:10.1016/j.jmb.2008.01.087 .
Source: Argonne National Laboratory















