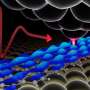
How molecular muscles help cells divide
Time-lapse videos and computer simulations provide the first concrete molecular explanation of how a cell flexes tiny muscle-like structures to pinch itself into two daughter cells at the end of each cell division, according to a report in Science Express.
Cell biologists at Yale and physicists at Columbia teamed up to model and then observe the way a cell assembles the “contractile ring,” the short-lived force-producing structure that physically divides cells and is always located precisely between the two daughter cell nuclei.
“This contractile ring is thought to operate like an old-fashioned purse string,” said senior author Thomas D. Pollard, Sterling Professor and Chair of the Department of Molecular, Cellular & Developmental Biology at Yale. “It constricts the cell membrane into a cleavage furrow that eventually pinches the cell in two.”
Living cells divide into two daughter cells to reproduce themselves. In one-celled organisms like yeast, each cell division yields a new creature. In humans and other multicellular species, cell division creates an adult from an embryo. In fully developed adults, it provides necessary replacements for cells that are continuously dying in the course of natural wear and tear.
Scientists have long studied aspects of how cells actually make this division — the structure of the cellular machinery, how it assembles and how the machine works. Since the 1970s, it has been known that the contractile ring is made up of muscle-like actin and myosin — contractile proteins that are involved a process in some ways similar to the muscle contraction used to move arms or legs. However, there was no plausible mechanism to explain how it worked.
“We found that fission yeast cells assemble their contractile ring using a ‘search, capture, pull and release’ mechanism,” said Pollard. “This is important because it shows for the first time how the contractile machinery assembles and how all the pieces get to the right place to get the job done.”
Time-lapse imaging and computer modeling demonstrated that cells undergoing mitosis set up small clusters of proteins, or nodes, on the inside of the cell membrane around the equator of the cell. Proteins in these nodes begin to put out a small number of filaments composed of the protein actin. The filaments grow in random directions until they encounter another node, where myosin motors in the contacted node pull on the actin filament, bringing the two nodes together.
However, the researchers found that each connection is broken in about 20 seconds. Releasing the connections and initiating subsequent rounds of “search and capture” appears essential to the assembly process, say the scientists. The assembly involves many episodes of attractions between pairs of nodes proceeding in parallel. Eventually the nodes form into a condensed contractile ring around the equator, ready to pinch the mother into two daughters at a later stage.
“A novel and important aspect of this work was that we used computer simulations at every step to test what is feasible physically and to guide our experiments,” said author Ben O’Shaughnessy, professor of chemical engineering at Columbia. “The simulations show that cells use reaction rates that are nearly ideal to make this mechanism work on the time scale of the events in the cells.”
“Future work will involve testing the concepts learned from fission yeast in other cells to learn if the mechanism is universal,” said Pollard. “Since other cells, including human cells, depend on similar proteins for cytokinesis [cell division], it is entirely possible that they use the same strategy.”
Source: Yale University