Mother-of-Pearl in Highest Resolution
Mother-of-pearl, also known as nacre, is not just an iridescent substance whose optical characteristics impress the observer and which is often used for jewellery. It is also an excellent material for working with. Nacre consists of 97 percent lime, but has a thousand times higher breaking strength. The reason has to do with the layer composition of mother-of-pearl. Now, Max Planck and BAM scientists have discovered that the surface of the lime platelets in mother-of-pearl is not at all ordered in layers, as had been previously assumed.
Because of this fact, it can be ruled out that the crystals are controlled through ordered layers on the organic matrix. This understanding of nacre, and the mechanism by which it is built, is essential for emulating the same refined principle in building new materials.
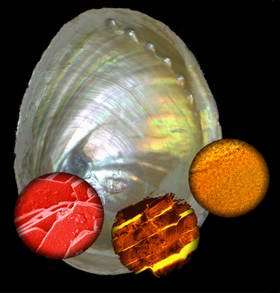
For a long time, mother-of-pearl has been considered as an interesting biogenous material. During this time, researchers have been trying to understand its astounding characteristics. Its unusual breaking strength is due to a structure based on soft organic layers and hard lime platelets. (Image 2)
If we could only begin to copy this building principle, it would lead to a revolution in the construction industry. Possible goals for this kind of biomimetic materials research could be firmer gypsum plasterboard, or pieces of concrete with a lower weight but with the same strength. The lime platelets in nacre crystallise into aragonite - a crystal form which is normally not stable under ambient conditions. Until now, researchers had assumed that this crystallization of the lime platelets was determined by ordered layers of protein which lie on a pre-formed layer of chitin. Chitin can be found in nature, as for example a scaffolding material in the shells of insects.

Image 2: Scanning electron microscope view of the fractured surface of nacre. (Max Planck Institute of Colloids and Interfaces)
But the latest findings of the Max Planck scientists have found these assumptions to be false. Instead of an ordered crystalline layer, which would be in contact with the organic matrix, the scientists found tiny - five nanometres thick - layers of amorphous - that, is disordered - calcium carbonate on the surface of the monocrystalline platelets in nacre.
This disordered and wavy surface provides evidence against the postulated specific interaction between the mineral material and the organic matrix. The finding could be clearly supported by 13C and 1H solid state nuclear magnetic resonance (NMR) spectroscopy. Furthermore, in NMR experiments the researchers detected the amorphous character of the surface layer and ruled out any interaction between it and the organic scaffolding.
The reason for the existence and development of the disordered upper layer on the crystal could be based upon the fact that impurities accumulate in the surface layer. In crystallization, these are not built into the ordered crystal lattice - similar to what happens in the process of zone melting in metallurgy.
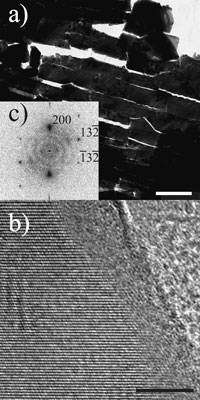
Image 3: (a) Transmission electron microscopy (TEM) - thin slices of aragonite platelets in nacre (scale: 1 micrometer) (b) High resolution TEM (HRTEM) of a selected surface of the monocrystalline aragonite platelets (scale: 5 nanometres). The disordered surface is recognizable next to the ordered crystalline layer with the stripe-shaped crystal lattice plane. (c) Power spectrum of the HRTEM image in (b). The crystal is oriented along direction [023] (Max Planck Institute of Colloids and Interfaces)
The amorphous layer (ACC) could indeed have another function. It replaces the previously assumed direct interaction of the high energy (001) aragonite layer through a gradient layer made of aragonite, ACC, and organic matrix. The energies of the boundary layer could be significantly lower here, and thus a thermodynamic force could exist for the development of an amorphous upper layer. It is still not clear in which direction the crystallographic orientation of the platelet eventually moves. In the current study, the scientists have acted on the assumption that an electrostatic attraction exists between the inorganic platelets and the organic matrix.
Original work:
Nadine Nassif, Nicola Pinna, Nicole Gehrke, Markus Antonietti, Christian Jäger, and Helmut Cölfen
Amorphous layer around aragonite platelets in nacre
PNAS 2005 102: 12653-12655; published online before print: August 29 2005, print: September 6, 2005, Vol. 102, No. 36
Source: Max Planck Society