Research advances understanding of how hydrogen fuel is made
Oxygen may be necessary for life, but it sure gets in the way of making hydrogen fuel cheaply and abundantly from a family of enzymes present in many microorganisms. Blocking oxygen’s path to an enzyme’s production machinery could lead to a renewable energy source that would generate only water as its waste product.
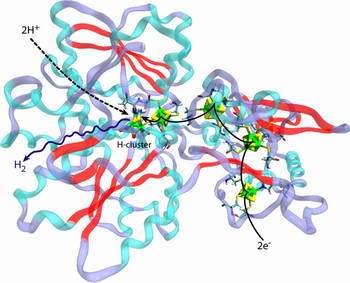
Image: Schematic diagram of hydrogen-oxygen reaction taking place in hydrogenase CpI. (Graphic courtesy of Jordi Cohen)
Researchers at the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign have opened a window by way of computer simulation that lets them see how and where hydrogen and oxygen travel to reach and exit an enzyme’s catalyst site – the H cluster – where the hydrogen is converted into energy.
The Illinois scientists and three colleagues from the National Renewable Energy Laboratory in Golden, Colo., detailed their findings in the September issue of the journal Structure. What they found could help solve a long-standing economics problem. Because oxygen permanently binds to hydrogen in the H cluster, the production of hydrogen gas is halted. As a result, the supply is short-lived.
Numerous microorganisms have enzymes known as hydrogenases that simply use sunlight and water to generate hydrogen-based energy.
“Understanding how oxygen reaches the active site will provide insight into how hydrogenase’s oxygen tolerance can be increased through protein engineering, and, in turn, make hydrogenase an economical source of hydrogen fuel,” said Klaus Schulten, Swanlund Professor of Physics at Illinois and leader of the Beckman’s Theoretical Biophysics Group.
Using computer modeling developed in Schulten’s lab – Nanoscale Molecular Dynamics (NAMD) and Visual Molecular Dynamics (VMD) – physics doctoral student Jordi Cohen created an all-atom simulation model based on the crystal structure of hydrogenase CpI from Clostridium pasteurianum.
This model allowed Cohen to visualize and track how oxygen and hydrogen travel to the hydrogenase’s catalytic site, where the gases bind, and what routes the molecules take as they exit. Using a new computing concept, he was able to describe gas diffusion through the protein and predict accurately the diffusion paths typically taken.
“What we discovered was surprising,” Schulten said. “Both hydrogen and oxygen diffuse through the protein rather quickly, yet, there are clear differences.”
Oxygen requires a bit more space compared with the lighter and smaller hydrogen, staying close to few well localized fluctuating channels. The hydrogen gas traveled more freely. Because the protein is more porous to hydrogen than to oxygen, the hydrogen diffused through the oxygen pathways but also through entirely new pathways closed to oxygen, the researchers discovered.
The researchers concluded that it could be possible to close the oxygen pathways of hydrogenase through genetic modification of the protein and, thereby, increase the tolerance of hydrogenases to oxygen without disrupting the release of hydrogen gas.
Co-authors with Schulten and Cohen were Kwiseon Kim, Paul King and Michael Seibert, all of the National Renewable Energy Laboratory. The National Institutes of Health, National Science Foundation and the U.S. Department of Energy funded the research.
NAMD is a parallel molecular dynamics code designed for high-performance simulation of large biomolecular systems. VMD is a molecular visualization program for displaying, animating and analyzing large biomolecular systems using 3-D graphics.
Source: University of Illinois at Urbana-Champaign
















